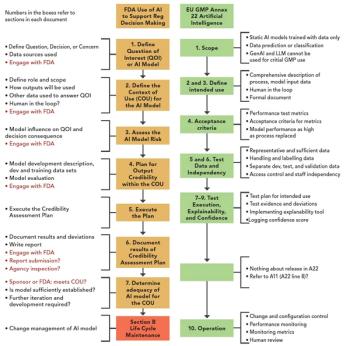
Does AI/ML help to enhance chromatographic peak integration? What do regulators say? Are you being seduced by technology?
R.D. McDowall is Director at R.D. McDowall Ltd, Bromley, Kent, UK. A company involved in process redesign, the specification, implementation and validation of computerized systems, laboratory digitalization, data integrity assessment and remediation, training in these areas and auditing regulated organisations in the pharmaceutical and allied industries. He is also a member of the LCGC International editorial advisory board (EAB).

Does AI/ML help to enhance chromatographic peak integration? What do regulators say? Are you being seduced by technology?

Twenty years ago, the fraud at Able Laboratories was uncovered. A chromatography data system (CDS) was at the center of the fraud. What have regulated laboratories learnt since then?

Protecting your network chromatography data system (CDS) data is critical and a service level agreement (SLA) with your IT provider is vital. What should be included? Are SLAs for in-house IT and SaaS (software as a service) similar?

In this column, we look at the current version and the update of USP <621> on high-performance liquid chromatography (HPLC) that becomes effective 1st May 2025.

Tired of life as your analytical results are always out of specification (OOS)? Fed up with yet another laboratory investigation? Get those rotten chromatograms to generate passing results by learning ways to manipulate peak integration from the experts... and now I have your undivided attention...and how reviewers, QA and inspectors can detect them!

“Questions of Quality” is 30 years old! What, if anything, has changed in chromatography laboratories over that time?

The scope of data integrity is shown by the data integrity model, which sits within the overall pharmaceutical quality system of a regulated laboratory.
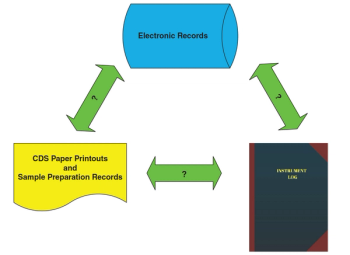
The term orphan data is used frequently in the context of data integrity. What does it mean for chromatography data systems? How can we prevent or detect orphan data?

What do the draft publications ICH Q2(R2) and Q14 for analytical procedure validation and development mean for a regulated GMP laboratory?

A balance printout is a fixed record, and is also called static data. But how static are static data when the weight is used in a chromatographic analysis? Also, have some regulatory data integrity guidance documents failed to comply with their own regulations?

Qualification and calibration of high performance liquid chromatography (HPLC) chromatographs is a regulatory requirement, but how proscriptive should guidance be?

There is a hidden factory in plain sight in many laboratories. Not many people know of its existence, yet it stares back at you when you work. What is the output from this hidden factory? Paper.

In September, the World Health Organization (WHO) issued a new guidance document on Good Chromatography Practices. What guidance does it contain and is it useful? Has the document failed its system suitability test (SST) acceptance criteria?

In September the World Health Organisation (WHO) issued a new guidance document on Good Chromatography Practices. What guidance does it contain and is it useful? Has the document failed its SST acceptance criteria?

This article uses case studies to explore the use and misuse of spreadsheet calculations in conjunction with a chromatography datasystem (CDS) in regulated GXP laboratories. What wonders of non-compliance will we find? How and when should spreadsheets be used in chromatographic analysis?

Are your OOS investigations scientifically sound and is the assignable cause correct? Or are OOS results invalidated using the ever-popular justification of analyst error?

Chromatography data systems (CDS) have been at the center of multiple FDA 483 citations and warning letters, with an emphasis on peak integration and interpretation of chromatograms. Here, we review the issues associated with ensuring compliance when performing peak integration.
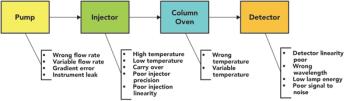
How should you maintain the calibration and qualification of chromatographs during the COVID-19 pandemic? Here, we discuss the details of how to ensure data integrity of results when faced with travel limitations and requirements for social distancing.

A key component for data integrity is having accurate and precise analytical procedures that are validated for intended use. Changes in the way that procedures are specified, developed, validated, and operated are coming. Here is what you need to know.
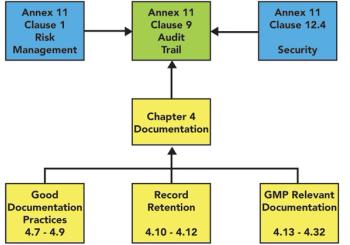
Examination of audit trail entries is a key part of the second-person review. What do regulatory documents say about this topic, and how can we speed up review?

Hybrid chromatography data systems are the worst possible solution for any laboratory, whether regulated or unregulated, and yet they are pervasive. Here’s why.

Chromatographic peak integration continues to be a major regulatory issue and was first discussed in this column in 2015. Is the approach to manual intervention and manual integration outlined still acceptable in the light of regulatory citations and guidance documents published since then?
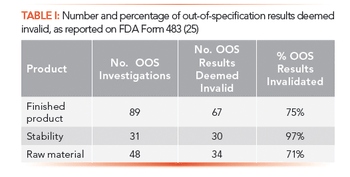
Here, we review the regulatory expectations of metrics for monitoring data integrity and discuss their practical implementation in a regulated chromatography laboratory.

Are EU GMP Chapter 4 and USP Chapter on Good Documentation Guidelines similar or complementary? Should we consider any other sources of advice?

Several regulatory data integrity guidance documents require that laboratories understand the data life cycle of their processes, but provide only vague definitions of what a data life cycle is. Here is a practical interpretation.

What are the roles and responsibilities of quality assurance staff for ensuring data integrity in an organization?

USP chapter can help ensure data integrity of a chromatography data system. Here’s how.

The meaning of the terms raw data and complete data are explored. One term is from EU GMPs and the other is from US GMPs. Do they mean the same thing?
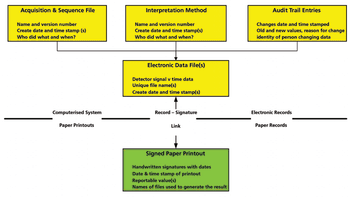
A hybrid system is the worst possible choice for managing your regulated data.

Data integrity is paramount when working in a regulated environment. Data process mapping is an excellent way to identify and mitigate data gaps and record vulnerabilities in a chromatographic process. This approach is simple and practical.

Published: September 1st 2023 | Updated:

Published: January 1st 2000 | Updated:

Published: September 16th 2024 | Updated:

Published: November 1st 2021 | Updated:

Published: October 1st 2022 | Updated:

Published: February 1st 2024 | Updated: