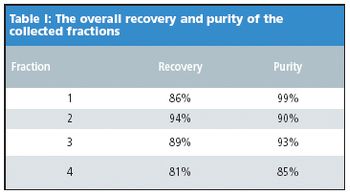
In the past decade, supercritical fluid chromatography (SFC) has experienced a striking resurgence and exponential growth in acceptance, particularly in pharmaceutical and chemical laboratories. In SFC, "supercritical" CO2, in combination with one or more polar organic solvents, most commonly alcohols, are used as mobile phase. The polarity of CO2 is similar to that of hexane, and thereby making SFC a normal phase chromatographic technique. SFC has readily lent itself as an attractive complement to reversed phase HPLC (RPLC). For instance, in separating polar compounds that have little retention, and/or selectivity, even with special polar group embedded columns, SFC holds a unique advantage over RPLC due to its normal phase separation mechanism.

