Ingenious Ways to Manipulate Peak Integration?
Tired of life as your analytical results are always out of specification (OOS)? Fed up with yet another laboratory investigation? Get those rotten chromatograms to generate passing results by learning ways to manipulate peak integration from the experts... and now I have your undivided attention...and how reviewers, QA and inspectors can detect them!
How a Chromatography data system (CDS) can be involved in data falsification in regulated good practice (GXP) environments is public knowledge since the 2005 Able Laboratories fraud case (1). Chromatograms were reintegrated multiple times, but the most creative falsification was chromatographic titration using this sequence file: sample weights were changed until a passing result was obtained (1). FDA missed the fraud (as they focused on paper records), but a whistleblower alerted the local field office, and the rest is history. Ironically, all data manipulation was recorded in the CDS audit trail. The Able fraud case has resulted in three updates of the FDA Compliance Policy Guide 7346.832 for Pre-Approval Inspections (PAIs) since 2010 (2–4); the last two updates were discussed in my “Focus on Quality” (“FOQ”) column in Spectroscopy (5,6).
A “Questions of Quality” (“QOQ”) column discussed the role of CDS in fraud and falsification and described the 10 compliance commandments (7). If implemented, these commandments should help prevent many poor data management practices such as shared user identities, roles with conflicts of interest, deleting records, shredding printouts, selective reporting, invalidating out of specification (OOS) results because of human error, manual integration, turning the audit trail off and back on, or failing to turn it on in the first place.
Let us see how the CDS Class of 2023 have failed when inspected by the FDA. I would like to thank Paul Smith for providing the 13 483 citations from 2023. I have extracted the CDS-related citations from the 483s, classified them, and lightly edited them to fit in Table I. Be warned that regulatory authorities have received training in CDS applications from software vendors.

The key question? Have companies learned and implemented earlier CDS compliance lessons? Spoiler alert...No! Given the attention that regulatory agencies have given chromatography since 2005, you would have thought that companies would have made some progress to eliminate common problems, but no. On the plus side, this provides me more data to highlight the stupidity of some organizations to keep current with guidance and regulatory actions. In this column, we look at the current ways of poor data management practices and data falsification including peak integration—again! This is the third time the subject has been discussed in a “Questions of Quality” column (8,9).
The focus of this column is on the CDS, but don’t forget that much of the preliminary sample preparation is manual, and, in principle, laboratories need to perform a risk assessment to determine if the process needs to be witnessed (10).
Warning Lights Are Flashing in Quality Control
Since Able, we have seen several ways chromatographers can attempt to pass product batches:
- Test and Prep Samples: The old school approach to testing into compliance created a directory called “Test, Wash, Demo or Prep,” and sample injections were made to see if the batch would pass or not (24–26). One such folder contained over 3300 data files (27). All are easy to detect, and this has been phased out in favor of other options to cheat. Ideally, your laboratory should only allow one location to store CDS data for any run.
- Conflicts of Interest: Another old school non-compliance that should have been resolved by now is to give users privileges where they can do activities they should not be able to do. Table I shows that the practice is not extinct, and users still can delete or manipulate data if they are given the privileges. My view is simple—no user should have deletion privileges.
- Audit Trail Not Turned On: Table I also has an instance where the system audit trail has not been turned on (12). Simple stupidity...either not thinking about data integrity, or not bothering to understand the CDS technical controls available versus the regulatory requirements (28,29).
- Selective Reporting or Lack of Complete Data: There are several citations for the lack of complete data in Table I. This can vary from only saving the original file and the “final” reintegrated file to ignoring failing results and only reporting results within the specification. Table I shows an example where a sample was reintegrated 33 times to obtain the correct result (15). Integrating into compliance?
- Hiding Peaks by Printing: One way to hide poor impurity peak shape is to print the main peak as 100% full scale deflection, allowing the impurities to disappear into the baseline. A reviewer would need an electron microscope to see the peaks on the printout. Ignore printouts and review chromatograms on screen, as discussed later.
- Instrument “Problems”: Has a chromatograph suffered a malfunction that invalidates the run? If so, the impacted chromatograms should have evidence of the problem; for example, a leaky piston seal should result in poor peak shape and longer retention times. Create a pump leak, then (shame!) the run must be invalidated and the analysis repeated so the batch passes. The falsified data hide in plain sight. Refer to the instrument logbook to see that the problem is documented, resolved along with any applicable requalification, and then refer to the CDS so as to check that the data file date and time stamps are consistent with the logbook entries. Table I has a real gem with instrument problems but also invalidating an OOS result: the data was invalidated as it was run on a system that does not provide the expected quality (13). The obvious question is: Why start an analysis using a chromatograph that was allegedly not fit for purpose? System suitability test (SST) acceptance criteria, anyone?
- Short or Aborted Runs: Another way to hide falsification is to use short or aborted runs of one or two injections using samples to see if they pass or not. Searches of the database should be able to identify short and aborted runs. The question, then, is: why was the run aborted? This must be linked with the instrument problems discussed above. We discuss how to evaluate if the system is ready to run later in this column using guidance from the FDA website.
- Manual Injection: Accessing the quality of samples by manually injecting “outside” of the CDS, using the control panel on the chromatograph.
- IT Help Desk: Another way that file deletion has occurred has been through logging requests and changes through the IT Help Desk, which may not necessarily have the same awareness of the regulatory impact of the changes. Does your laboratory have a quality agreement with IT (28)? The FDA have audited IT Help Desk systems to check for this.
To highlight both instrument problems and short or aborted runs, a 483 citation for Aurobindo noted that the message center of a CDS logged 6337 (yes, really!) error messages from July 1 to August 1, 2022 (30). Analysis identified the following:
- 411 instrument failure messages;
- 13 messages of sequence stopped because of error or sequence stopped by user; and
- 20 failed to get the newest information of the batch queue because of the communication failure messages.
For further reading, a “QOQ” column discusses orphan data (31), and an “FOQ“ column looked at the role of an instrument logbook (32).
The remainder of this column discusses peak integration and cover procedures, including failure to follow one and the use of various integration parameters to help or hinder your journey to data integrity nirvana.
Controlling Peak Integration
My experience is based on small molecule analysis, so please interpret my comments for more complex separations. There seems to be a great deal of confusion over terminology, in regards to what labels to use for different kinds of integration “activity” and what is allowed and not allowed. Earlier “QOQ” columns discussed the requirement for automatic peak integration to be applied to all chromatograms the first time, every time (8,9). It also differentiated manual intervention (changing automatic integration parameters with automatic baseline placement) from manual integration (manual reportioning of the baselines) (8). Manual intervention should be applicable to any chromatogram (such as for peaks outside expected retention windows, or increasing minimum area to reduce the impact of a noisy baseline), but the changes must be applied to all files in the sequence. From quality and regulatory perspectives, testing samples from a validated manufacturing process on a qualified instrument using a validated analytical procedure raises the question—why would you need to change the integration? This is why changes to integration for product assay methods would be interpreted suspiciously. Manual integration must only be allowed in specific cases such as impurities or complex separations, with the rationale documented in the method validation report.
As part of the laboratory controls, you need a procedure and training in peak integration. One citation in Table I stated: No procedure to describe how to correctly perform integration to ensure consistency from analyst to analyst and from day to day (15). It is not just having a procedure; it is about how a laboratory ensures consistency across the whole user base.
- Peak Integration Standard Operating Procedure (SOP): It is imperative that there is an SOP for peak integration. You have got one, haven’t you? Some FDA warning letters required laboratories to have an SOP for manual integration. In my view, this is wrong and is not scientifically sound as required by 21 CFR 211.160(b) (33). An imperative is an SOP that covers all peak integration, highlighting what must be done and especially what must not be done. It is not a treatise on the fundamentals of integration, and the book by Dyson (34) is the best source of integration knowledge. Where permitted, all integration changes made by an analyst must be justified and documented, typically in the audit trail. CDS applications provide the means to have user-defined reasons for change, and this feature should be used. The journey from the first to the last integration of any file must be traceable, complete, and with reasons for change to ensure data integrity. The peak integration SOP must be linked to individual analytical procedures where specific integration requirements must be defined.
- Peak Integration Training: What training in the integration procedure was carried out and what were the results? It is not just training individual chromatographers; it is also ensuring integration consistency throughout the laboratory. One laboratory has a series of standard chromatograms that all must integrate after training. Interestingly, results for assay had excellent agreement across the laboratory, but could vary with impurities close to the limits of quantification. Using automatic integration first time, every time, resolved much of the problem.
- Analysts Reviewing Other’s Work: This is important so that they gain experience of reviewing chromatographic data from an independent quality perspective and therefore better understand the risks and implications for changing integration.
You Have an SOP – Follow It!
It’s all very well having a procedure with effective training, but does a laboratory follow the SOP? The obvious answer is yes. Again, there are two examples in Table I where the integration SOP has not been followed (11, 14). In addition, there is another citation:
Procedure SE/BQC/00165 Interpretation of Chromatograms requires manual integration be documented clearly stating the reason the manual integration was performed and the initials of the section head for approval. But when analysts manually enter integration events to force the software to integrate in a specific way, there is no similar documented justification and approval process ... (35).
As the old saying goes, you can take a horse to water …..
Linking Integration Events to an Analytical Procedure
Closely allied with an integration SOP is the necessity to incorporate any pertinent integration requirements into each analytical procedure to avoid citations such as:
The method does not include how to properly apply these <redacted> integration events to process the analytical data and no procedure was provided on how to report <redacted> integration within the result sets (18).
Figure 1 shows a peak integration SOP that provides overall control of integration and what is allowed, what is not allowed, and what is applicable throughout the analytical procedure lifecycle (36). However, such a procedure cannot go into detail for all chromatographic methods, and this is the role of each analytical procedure:
- If the procedure is for an assay, can manual integration be performed at all? Many would argue that a method is out of control if you have to manually integrate the main peak.
- Example chromatograms should also include any specific requirements for integration parameters such as integrate inhibit and baseline zero, and be scientifically sound and justified. As shown in Figure 1, these parameters should be traceable to the development and validation reports.
- Only test “equivalent” samples on the same chromatographic run—in particular, segregate the analysis of stability samples from runs containing current production samples. Integration requirements and the impurity profile of the stability samples may mean different integration parameters are required.
FIGURE 1: Ensure specific integration requirements are incorporated in an analytical procedure and are traceable.
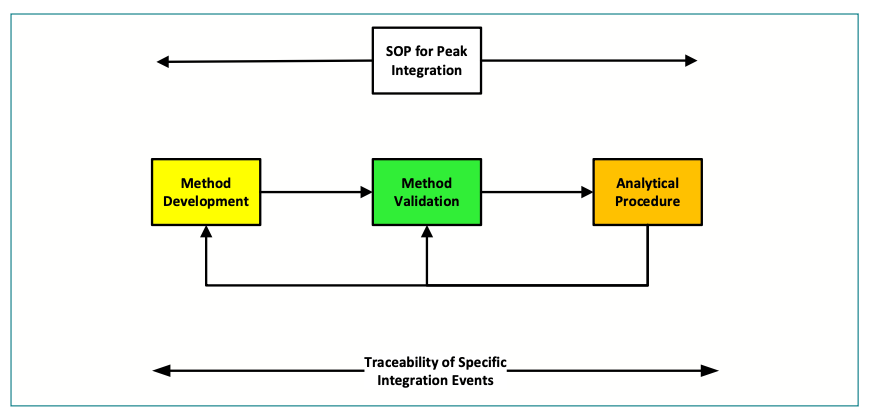
To help scientific justification, any integration parameters from the analytical procedure should be traceable back through the validation report to the development report shown in Figure 1. You know it makes sense, but will you be allowed time to do this? Also shown in Figure 1 are the return loops from the analytical procedure to development and validation phases of the lifecycle. If there is a problem with the analytical procedure, modification and revalidation may be required.
Cunning Chromatographers and Insidious Integration
Always remember that chromatography is a comparative and not absolute analytical technique. Therefore, all injections in a run must be integrated with the same method. Table I has an example where three processing different methods were used for the standard samples and yet another one for the samples (23).
This fact provides more subtle means of data manipulation by treating samples differently from standards. Reviewers must be aware of this and ensure that any attempts to manipulate peak integration is identified before results are calculated.
A more insidious approach was identified in the Intas Pharmaceuticals 483 (35):
... Additionally, the 6-month accelerated time point for the same lot <redacted> was integrated manually by adding a fronting sensitivity and a tailing sensitivity factor to the peak for impurity <redacted> but not for the standard of the same impurity. This reduced the area of the impurity compared and gave a result of <redacted>% compared to a limit of <redacted>%. When the fronting and tailing sensitivity factors are removed to ensure integration of the impurity compared with the standard, the reportable result changes to <redacted>%, a value that would have required an investigation.
This is one 483 citation—there are several other integration issues in the 483 (35).
Reviewers and quality assurance (QA) must be aware that standards and samples must be integrated using the same parameters and check to ensure that this has occurred.
System Suitability Test Failures
A short diversion is required to discuss the FDA’s present to the industry in the Guidance on Investigation of Out Of Specification Results. Under the “Responsibilities of the Analyst” section, there is the following:
Certain analytical methods have system suitability requirements, and systems not meeting these requirements should not be used. For example, in chromatographic systems, reference standard solutions may be injected at intervals throughout chromatographic runs to measure drift, noise, and repeatability. If reference standard responses indicate that the system is not functioning properly, all of the data collected during the suspect time period should be properly identified and should not be used. The cause of the malfunction should be identified and, if possible, corrected before a decision is made whether to use any data prior to the suspect period (37,38).
This is key to some peak integration problems and interprets the section above in a way the FDA did not intend. This also links with the instrument problems discussed earlier in this column. If the SSTs fail throughout the run, the files are still part of the complete data of the analysis but should not be used. Complete data (21 CFR 211.194(a) (33) is the regulatory requirement and the term that was discussed in earlier article (39). What most analysts forget is that the reason for the problem must be investigated and corrected.
How does this section impact peak integration malpractice? One way to invalidate a run is to integrate the SST injections to ensure that the acceptance criteria are out of limits. The material to use for SST injections is discussed on the FDA’s web site in the section on Laboratory Controls, Question 16 (40).
Is My Chromatogram Big Enough?
Second person review is critical to confirm chromatography has been performed correctly and that there is no data manipulation. To achieve this, forget printing chromatograms. Instead, implement electronic signatures, and, if you really must, only print a summary of the analysis. You must review chromatograms and the associated metadata, including audit trail entries on screen. Please understand that this section should not be used to justify the purchase of a 55-inch 4k internet-enabled screen, but you do need to have a large enough screen or dual screens to perform an adequate second person review. Have the chromatograms in one window and the audit trail or other run information in another so that you can cross reference both easily.
One function of a CDS that is critical for review, audit, or inspection is chromatogram overlay. Chromatograms can be plotted superimposed on each other to assess retention time and peak shape consistency throughout the run. Alternatively, to get a better picture, especially for impurities, the overlay offset function enables an easier comparison. A larger screen or screens enables the chromatograms and the applicable audit trail entries in a second window to be correlated simultaneously, rather than laboriously switching between windows with a small single screen.
Five Rules of Peak Integration
An integration SOP was discussed earlier to help understand what should be in it and the associated training. There are five rules to consider (9), summarized here:
- Rule 1: The main function of a CDS is not to correct your poor chromatography.
Good chromatography requires a robust analytical procedure with good peak shape and separation. Know and control the factors that influence separation and ensure that automatic peak integration is the norm not the exception. This is especially true for pharmacopoeial methods that never work as written. See Stage 1 of the USP <1220> [36] rather than the abysmal ICH Q2(R2) and Q14 guidance document [41, 42] that are not integrated and have large gaps in the final versions that were not corrected from the draft versions [43].
- Rule 2: Never use default integration parameters.
Using a default or generic method results in an excessive need for manual integration to name and calculate peaks. Without exception, peak integration and result processing must be defined and validated for each method so that all peak windows and names are established. Where necessary, any system peaks are identified. If used, integrate inhibit must be scientifically justified and be traceable to method development and validation reports. Unlike Dr Reddy’s, who received a 483 citation for the incorrect use of the integrate inhibit function to mask an unknown peak in samples that was not present in blank or standard samples coupled with no investigation of the problem (22).
- Rule 3: Always use automatic integration as a first option and control manual integration.
Remember that the use of manual integration is a regulatory concern, and its use needs to be scientifically sound. Manual integration slows down processing, so see Rule 1 to get the right method, depending on the sample matrix and peaks of interest.
- Rule 4: Understand how the CDS works and how the numbers are generated.
This requires basic training in the principles of peak integration and how a CDS works. The problem is that with company mergers or acquisitions that encourage experienced analysts to retire and employ younger workers, skills are being eroded, and a CDS can be looked at as a black box that always gives the right answers. Learn and understand how the basics of a CDS works.
- Rule 5: Use your brain—think.
This rule is sometimes difficult to follow, but it builds on Rule 4. You can have what appears to be a perfect separation and peak integration, but look at the peak start and end placement; do they look right? Does there appear to be a coeluted peak? Use the zoom and overlay functions of the CDS to see if the standards and samples have the right peak shape. The analyst has the responsibility to execute applicable procedures correctly, which includes correct peak integration. The reviewer also has a role to ensure that all integration (whether automated or manually placed) follows the guidance for placing baselines as the SOP and analytical procedure describe. Significant peak area manipulation should be easily noticed by an experienced reviewer.
Can I Use System Evaluation Injections?
Good scientific sense is to check that the chromatograph and column is equilibrated and ready for analysis. However, some laboratories are fearful of doing this, as they might be accused of testing into compliance with sample injections. Help is at hand from an FDA Q&A on laboratory controls on their website (40). Question 17: Is it ever appropriate to perform a “trial injection” of samples?
- No.
This is unofficial testing disguising testing into compliance which is a violation of GMP and is unacceptable.
- Column conditioning does not involve injecting a sample from a lot and is not considered a trial injection. When its use is scientifically justified, column conditioning should be fully described in the method validation package as to the conditions needed to make the measurement (that is, based on data from the method validation) and should be clearly defined in an approved and appropriate procedure.
- Consistent and unambiguous injection nomenclature should be used, and all data from the column conditioning, including audit trail data, should be maintained and subject to review.
Note the wording in the second bullet point that the use of conditioning injections should be traceable to the method validation or verification report with criteria to determine if the system is ready to start analysis. You must use the correct terminology for injections and conditioning injections (not Test or Prep!), as they are part of complete data (33) or raw data (44) for any run.
Will AI Solve My Integration Problems?
I know what some of you are thinking, that all I have written here is tosh, and artificial intelligence will solve all my problems. In your dreams; the clue is in the name intelligence. You must train an AI application, and this means you must know what you and the CDS are doing (see Rules 4 and 5), and you need good chromatography for good integration (see Rule 1).
Workman has provided a good overview of the background and essentials of AI in analytical chemistry (45). Trawling the internet will not capture a scientifically sound integration method for a specific separation. Different CDS applications have different algorithms for peak integration, and, while you may get a separation on one system, another one may integrate the same chromatogram differently; peaks areas could be the same, but rarely identical. It is imperative that you have good quality data sets for training the AI application (46).
You may be better off waiting until your CDS supplier has developed an AI module for you to train to help you integrate peaks.
Summary
Chromatography data systems continue to be the source of many poor data management and falsification practices found in regulated laboratories. Often, these are repetition of poor practices that are well known, and steps should have been taken to avoid them. Ensuring the data quality and data integrity relies on culture and ethics as well as a procedure and training for peak integration. Reviewers, QA, auditors, and inspectors are aware of these, and they will be checking them. Looking on the bright side, failure to learn or improve will be a continuing source of future “Questions of Quality” columns.
Acknowledgement
I would like to thank Paul Smith for providing the 13 483 citations that form the basis of this column and for his review comments.
References
(1) Able Laboratories Form 483 Observations. 2005, Available from:https://www.fda.gov/media/70711/download
(2) Compliance Program Guide 7346.832 Pre-Approval Inspections. 2010, Food and Drug Administration: Silver Springs ,MD.
(3) Compliance Program Guide CPG 7346.832 Pre-Approval Inspections. 2019, Food and Drug Administration: Sliver Spring, MD.
(4) Compliance Program Guide CPG 7346.832 Pre-Approval Inspections. 2022, Food and Drug Administration: Silver Spring. MD.
(5) McDowall, R. D. Do You Understand the FDA’s Updated Approach to Pre-Approval Inspections? Spectroscopy 2019, 34 (12), 14–19.
(6) McDowall, R. D. Guess Who’s Coming to Inspect R&D? Spectroscopy 2023. 38 (9), 6–10. DOI: 10.56530/spectroscopy.cv2490n6
(7) McDowall, R. D. The Role of Chromatography Data Systems in Fraud and Falsification. LCGC Europe 2014, 27 (9), 486–492.
(8) McDowall, R. D. Where Can I Draw The Line? LCGC Europe 2015, 28 (6), 336–342.
(9) Longden, H.; McDowall, R. D. Can We Continue to Draw the Line? LCGC Europe 2019, 21 (12), 641–651.
(10) MHRA GXP Data Integrity Guidance and Definitions. 2018, Medicines and Healthcare Products Regulatory Agency, London, England.
(11) FDA 483 Observations, International Trading Pharm Lab Inc., 2023.
(12) FDA 483 Observations, Kayserberg Pharmaceuticals, S.A.S., 2023.
(13) FDA 483 Observations, AmbioPharm Inc., 2023.
(14) FDA 483 Observations, Regeneron Ireland Designated Activity Company, 2023.
(15) FDA 483 Observations, Mylan Laboratories Limited (SFF), 2023.
(16) FDA 483 Observations, PEL Healthcare LLC.,2023.
(17) FDA 483 Observations, Cetylite Industries Inc., 2023.
(18) FDA 483 Observations, Patheon Biologics LLC,. 2023.
(19) FDA 483 Observations, Wisconsin Medical Radiopharmacy, LLC. 2023.
(20) FDA 483 Observations, Terumo Corporation, 2023.
(21) FDA 483 Observations, Centaur Pharmaceuticals Private Limited, 2023.
(22) FDA 483 Observations, Dr Reddy’s Laboratories Limited, 2023.
(23) FDA 483 Observations, IPCA Laboratories Limited., 2023.
(24) FDA Warning Letter Fresenius Kabi Oncology (WL: 320-13-20). 2013, Food and Drug Administration, Silver Springs, MD.
(25) FDA Warning Letter Wockhardt Limited (WL 320-13-21). 2013, Food and Drug Administration: Silver Springs, MD.
(26) FDA Warning Letter Wockhardt, India, FEI 3002808500., 2016, Food and Drug Administration: Silver Spring, MD.
(27) FDA Warning Letter Apotex Research Pvt Ltd., 2015, Available from: http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm432709.htm
(28) EudraLex - Volume 4 Good Manufacturing Practice (GMP) Guidelines, Annex 11 Computerised Systems. 2011, European Commission: Brussels, Belgium.
(29) 21 CFR Part 11; Electronic Records; Electronic Signatures Final Rule. Federal Register, 1997, 62 (54), 13430–13466.
(30) FDA 483 Observation Aurobindo Pharma Limited, Unit X1 August 2022. 2022, Food and Drug Administration, Silver Spring, MD.
(31) McDowall, R. D. What Exactly Are Orphan Data? LCGC Europe 2022, 35 (9), 381–387.
(32) McDowall, R. D. The Humble Instrument Log Book. Spectroscopy 2017, 32 (12), 8–12.
(33) 21 CFR 211 Current Good Manufacturing Practice for Finished Pharmaceutical Products. 2008, Food and Drug Administration, Sliver Spring, MD.
(34) Dyson, N. Chromatographic Integration Methods. 2nd ed.; 1998, Royal Society of Chemistry.
(35) Intas Pharmaceuticals Limited Form 483 Observations. Available from: https://www.fda.gov/media/164602/download. 2022.
(36) USP General Chapter <1220> Analytical Procedure Lifecycle. 2022, United States Pharmacopoeia Convention Inc:, Rockville, MD.
(37) FDA Guidance for Industry Out of Specification Results. 2006, Food and Drug Administration, Rockville, MD.
(38) FDA Guidance for Industry, Investigating Out-of-Specification (OOS) Test Results for Pharmaceutical Production. 2022, Food and Drug Administration, Silver Spring. MD.
(39) McDowall, R. D. Data Integrity Focus IV: Are Raw Data and Complete Data the Same? LCGC N.Amer. 2019, 37 (4), 265–268.
(40) FDA Questions and Answers on Current Good Manufacturing Practices—Laboratory Controls: Level 2 Guidance. Available from: https://www.fda.gov/drugs/guidances-drugs/questions-and-answers-current-good-manufacturing-practices-laboratory-controls
(41) ICH Q2(R2) Validation of Analytical Procedures, Step 4 Final. 2023, International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH): Geneva, Switzerland.
(42) ICH Q14 Analytical Procedure Development. Step 4 final. 2023, International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH): Geneva, Switzerland.
(43) Burgess, C.; McDowall, R. D. Quo Vadis Analytical Procedure Development and Validation? Spectroscopy 2022, 37 (9), 8–14.
(44) EudraLex - Volume 4 Good Manufacturing Practice (GMP) Guidelines, Chapter 4 Documentation, E. Commission, Editor. 2011, Brussels, Belgium.
(45) Workman J. Exploring Artificial Intelligence in Analytical Chemistry. 2023, Available from: https://www.chromatographyonline.com/view/exploring-artificial-intelligence-in-analytical-chemistry
(46) Hayes, M. A. Becoming an AI Grandaddy 2023, Available from: https://www.chromatographyonline.com/view/becoming-an-ai-granddaddy
ABOUT THE COLUMN EDITOR
Bob McDowall is Director at R.D. McDowall Ltd, Bromley, Kent, UK. A company involved in process redesign, the specification, implementation and validation of computerised systems, laboratory digitalisation, data integrity assessment and remediation, training in these areas and auditing regulated organisations in the pharmaceutical and allied industries. He is also a member of LCGC International editorial advisory board (EAB).


What Goes in a CDS IT Service Level Agreement?
Published: April 7th 2025 | Updated: April 7th 2025Protecting your network chromatography data system (CDS) data is critical and a service level agreement (SLA) with your IT provider is vital. What should be included? Are SLAs for in-house IT and SaaS (software as a service) similar?
Rethinking Chromatography Workflows with AI and Machine Learning
April 1st 2025Interest in applying artificial intelligence (AI) and machine learning (ML) to chromatography is greater than ever. In this article, we discuss data-related barriers to accomplishing this goal and how rethinking chromatography data systems can overcome them.
Leveraging an Enterprise Laboratory Informatics Platform to Maximize Scientific Data Advantage
September 9th 2024As data volumes and expectations for fast scientific discovery continue to increase, laboratory-based research organizations can no longer rely on a siloed approach to data management. To remain competitive, scientific organizations need to connect all their data, from discovery through manufacturing, in a unified informatics platform.
Advances in Chromatography Using Artificial Intelligence and Machine Learning
July 3rd 2024Scientists from the University of Turin, Italy have learned how to combine their complementary competencies in analytical chemistry and big data analytics to achieve significant advances in food science and health.