Pharmaceutical Analysis
Latest News
Latest Videos

More News

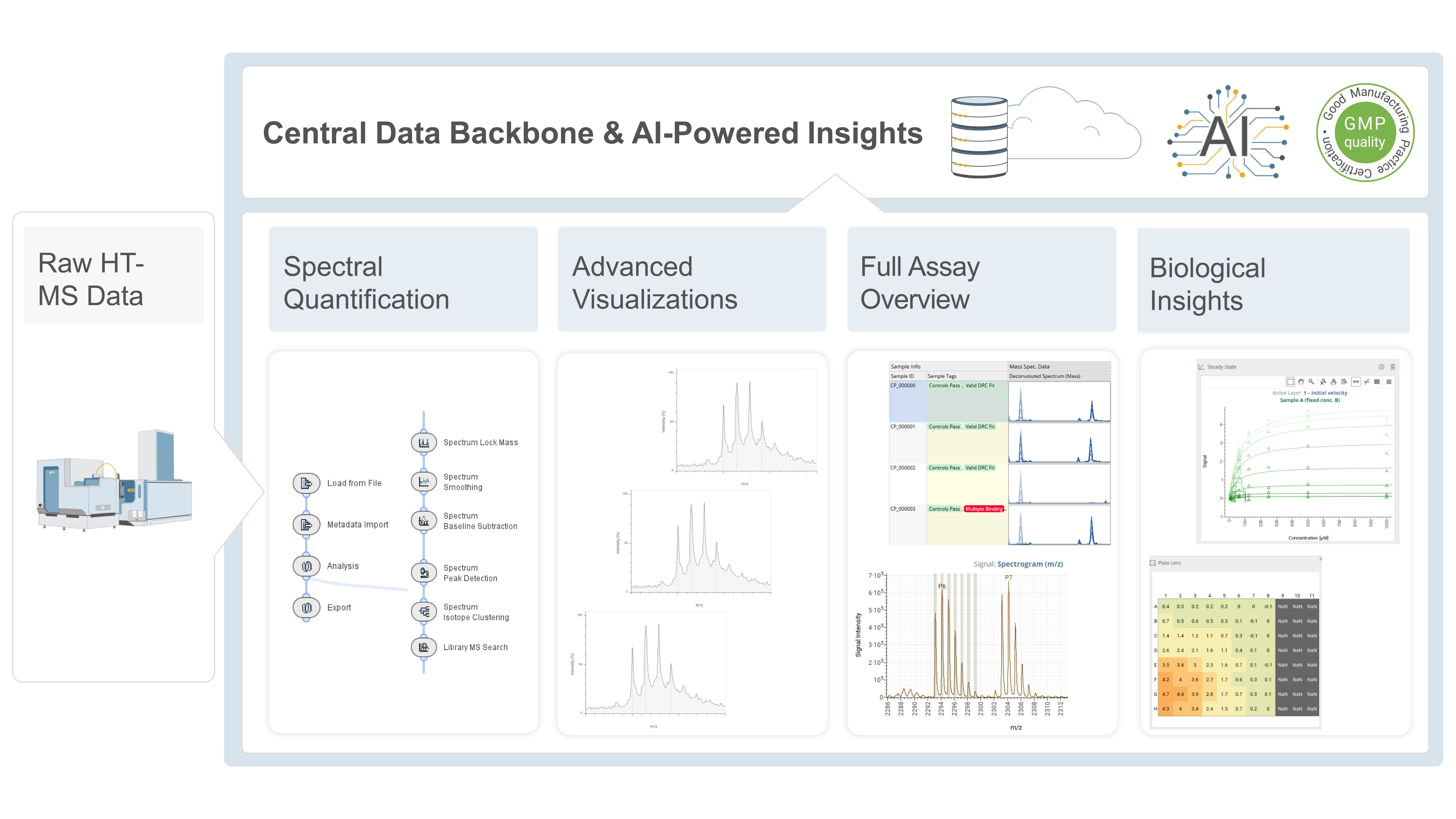
Key aspects involved in manufacturing high-quality packed capillary columns, including fritting, column packing, and parallel fabrication, are discussed.

HPLC is indispensable in pharmaceutical analysis and quality control despite some notable shortcomings in laboratory testing and processes to ensure regulatory compliance.

SFC Europe 2025—the 19th International Conference on Packed Column Supercritical Fluid Chromatography and Related Techniques—will be held in Basel, Switzerland, on October 5–7, 2025. LCGC International spoke to John Reilly, Maria Kristina Parr, and Larry Miller about the important role that SFC now plays in life science analysis

SFC Europe 2025—the 19th International Conference on Packed Column Supercritical Fluid Chromatography and Related Techniques—will be held in Basel, Switzerland, on October 5–7, 2025. LCGC International spoke to John Reilly, Maria Kristina Parr, and Larry Miller about the evolution of supercritical fluid chromatography (SFC) in terms of technology and applications.

Analytical instrument makers reported strong Q2 2025 results, driven by sustained demand in the pharmaceutical and chemical industries, with consumables and recurring revenues outpacing instrument sales.

In this article, you’ll find some of the top content that was published on LCGC this week, including calls for new chromatography technologies and interviews with rising stars in the field.

In this article, you’ll find some of the top content that was published on LCGC this week, including our upcoming forensic science content series and an interview on preanalytical factors for ethanol testing.

The company plans to invest billions in a new drug manufacturing hub in Virginia, dedicated to chronic disease treatments, alongside additional facilities in California, Indiana, Maryland, Massachusetts, and Texas.

The pharmaceutical industry’s move toward increased U.S.-based manufacturing is changing the landscape of career opportunities for analytical chemists.

In this article, you’ll find some of the top content that was published on LCGC this week, including a technical article on food flavor and an interview with the president of Shimadzu Europa.

In a new study, researchers used GC–MS and LC–MS were used to detect composition fraud in food supplement creation.

This article explores the use of chromatographic retention measurements in both LC and SFC to develop quantitative retention activity relationship (QRAR) models, offering a predictive tool for skin permeability without animal testing.

Discover a generic gas chromatography method for efficiently analyzing residual solvents in pharmaceuticals, ensuring compliance with regulatory standards.

Here is some of the most-read content posted on LCGC International this week.

Here is some of the most-read content posted on LCGC International this week.

Webinar Date/Time: Thu, Jun 26, 2025 11:00 AM EDT

Webinar Date/Time: Mon, Jun 9, 2025 11:00 AM EDT

Webinar Date/Time: Tue, Jun 17, 2025 11:00 AM EDT

Isavuconazole, an antimycotic agent used to treat fungal infections, can typically be found during dried blood spot sampling. However, there are obstacles that keep it from being an ideal approach for properly determining the drug’s presence.

Michael Dong recently discussed sample preparation (SP) techniques for small-molecule drug substances and solid dosage forms.

Webinar Date/Time: Wednesday, May 28, and Thursday, May 29, 2025 Morning Sessions: 2:00pm BST | 9:00am EST | 3:00pm CEST Lunchtime Sessions: 5:30pm BST | 12:30pm EST | 6:30pm CEST Afternoon Sessions: 6:30pm BST | 1:30pm EST | 7:30pm CEST

During a recent LCGC International peer exchange discussion on nitrosamine analysis, our professional panelists discussed the complex issues surrounding the processes for detecting nitrosamines.

LCGC International spoke with Daniel Meston and Dwight Stoll from Gustavus Adolphus College in St. Peter, Minnesota, USA, about a project they worked on with Todd Maloney from Eli Lilly in Indianapolis, Indiana, USA, to investigate the optimal performance conditions of antisense oligonucleotides (ASOs) when using hydrophilic interaction liquid chromatography (HILIC).

This article discusses several practical examples using bioinert columns, demonstrating their ability to deliver reliable, high-resolution results with precision and reproducibility.
A new method has been created for determining N-nitrosamines (NAs) in pharmaceutical preparations using liquid chromatography with tandem mass spectrometry.