Quo Vadis Analytical Procedure Development and Validation?
What do the draft publications ICH Q2(R2) and Q14 for analytical procedure validation and development mean for a regulated good manufacturing practice (GMP) laboratory? Are they consistent with the approach taken by the United States Pharmacopeia (USP) <1220> on Analytical Procedure Life Cycle? Why does it take the ICH two documents to describe what the USP can do in one?
Good analytical science, as well as ensuring data integrity, requires that analytical procedures should be validated for their intended use (1,2). The applicable Food and Drug Administration (FDA) good manufacturing practice (GMP) clause is 21 CFR 211.194(a)(2), which has been in the regulation since 1978 (3):
Similarly, EU GMP Chapter 6.15 on Quality Control requires validation of test methods (4):
Similar to instruments and systems that must be fit for intended use, so must analytical procedures be fit for intended purpose. However, how many readers define what the intended purpose of their analytical procedure must be?
These regulations apply equally to both in-house developed and pharmacopoeial methods. However, originally there was no regulatory guidance on how validation or verification was to be achieved. In this column, we will take you on a journey covering guidance for the validation of analytical procedures over the past 30 years. The starting point for this journey is to look in the sin bin of non‑compliances related to analytical results.
Does Your Analytical Procedure Generate OOS Results?
One of the continuing sources of schadenfreude with FDA warning letters and 483 observations is the invalidation of out of specification (OOS) results for a variety of unscientific reasons, such as the ever-popular “human error”—often without a scientific investigation. This results in invalidation of the result. This has resulted in the FDA generating a quality metric for any quality control (QC) laboratory of a percentage of invalidated OOS results (5), as well as a revision of the guidance on investigating OOS results in May 2022 (6).
Why are there many OOS results in some laboratories? After all, pharmacopoeial procedures always work as written? Right? Wrong!
Could a laboratory have rushed to validate a hastily developed procedure with the result that a poor procedure is the root cause of OOS results? We need a robust method development phase for any analytical procedure to have a sound foundation for validation and to ensure that OOS results are rare.
ICH and USP Approaches to Procedure Validation
We will focus mainly on publications from two organizations: United States Pharmacopeia (USP) and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) for analytical procedure validation. For those who are unaware, ICH was initially called the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use from its inception in 1990. It was a consortium of the regulatory authorities and pharmaceutical industry bodies from the US, Japan, and Europe. The aim was to develop unified approaches to key regulatory topics so that the pharmaceutical industry only had to perform one activity and it was acceptable in the three regions. Since then, additional regulatory authorities have joined (for example, Brazil and South Korea) and the name of the organization has changed. Conference has been replaced by Council, but it is still known as ICH.
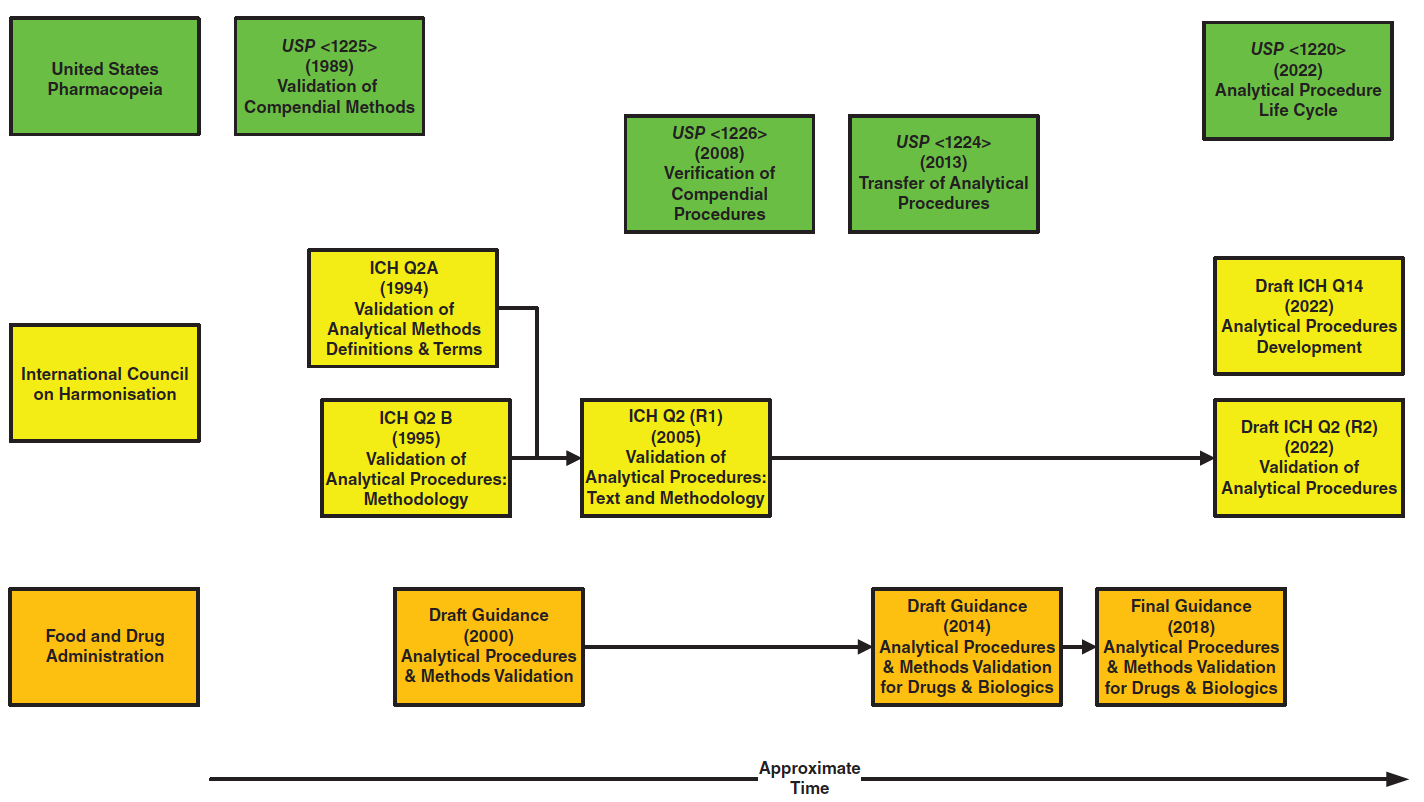
As you can see in Figure 1, the FDA have also published two draft and one final guidance document on validation for analytical procedures and methods. Which brings us to an interesting question: procedure or method?
Analytical Method or Analytical Procedure?
It is important to understand what is meant by analytical procedure and if there is a difference with analytical method:
- Analytical procedure refers to the whole process, from sampling through to reporting the result.
- Analytical method usually only refers to the instrument portion or analytical principle/separation technique of the analytical procedure.
Therefore, analytical procedure is the preferred term, as there may be problems when sampling (light sensitive analyte) during preparation of the sample dilution/extraction before analysis or during transport or storage (unstable analyte), which will not be considered if you are focused on the analytical method. Unless you are the FDA, who have bet on both terms.
In The Beginning—1: Initial Validation Guidance
Although 21 CFR 211 was published in 1978, there was no official guidance available on how to validate an analytical procedure; eventually the USP issued an informational general chapter <1225> entitled Validation of Compendial Methods in 1989 (7).
In addition to general chapter <1225>, USP has also issued two more general chapters:
- USP <1226> Verification of Compendial Procedures (8)
- USP <1224> Transfer of Analytical Procedures (9).
In the Beginning—2: ICH Q2
USP <1225> served as the foundation for the development of ICH Q2 Guidance on Validation of Analytical Procedures, which was published in two parts:
- ICH Q2A Validation of Analytical Methods: Definitions and Terminology in 1994 and is the parent guideline;
- ICH Q2B Step 4 Validation of Analytical Procedures: Methodology in 1996.
In 2005, ICH Q2A was renamed ICH Q2(R1) Validation of Analytical Procedures: Text and Methodology and incorporated into ICH Q2B, with no changes to the content of the combined document (10) (see Figure 1).
However, there are some problems with Q2:
- Procedure development is ignored; this is vitally important as cobbling a procedure together can result in variability, contributing to OOS results.
- Q2(R1) can be followed slavishly; for example, an analytical procedure for measuring assay between 90 and 110% of label claim often have limits of quantification (LOQ) and limits of detection (LOD) determined in the validation. The reason? It is in ICH Q2(R1)! This is stupid; sound science has not been applied, as required by 21 CFR 211.160(b) (3). We bet you didn’t know that a regulation required you to use sound science.
- The focus is mainly on chromatographic analysis.
In The Beginning—3: USP Life Cycle of Analytical Procedures
However, our interest lies in the latest USP general chapter <1220> on Analytical Procedure Life Cycle (11), which became effective on 1 May 2022. Bet you didn’t know that either! USP <1220> has had a long gestation; starting in 2011 it released the first stimulus paper to the Revision Process in 2012 (12), with further stimuli articles (13–15) and two draft versions <1220> (16,17) to support the development of the general chapter.
The life cycle approach is now a regulatory expectation and features
in ICH Q8 Pharmaceutical Development (18) and ICH Q12 Technical and Regulatory Considerations for Pharmaceutical Product Life Cycle Management (19). The aim of ICH Q8 is to move from quality by testing to quality by design (QbD), using knowledge and science as well as statistical design to achieve more robust pharmaceutical products. QbD is defined by ICH Q8 (18) as:
The key points are:
- Predefined objectives
- Product and process
understanding - Sound science
- Risk management.

These four points are incorporated in the three-stage life cycle of <1220> (11), which is shown in Figure 2 and comprises:
1. Procedure Design and Development (PPD): PPD or method development is based on the analytical target profile (ATP) for the procedure. Within this phase of the life cycle, the critical parameters are identified and risk-based ways to control them and an analytical control strategy defined. A design space is defined and tested using experimental design software.
2. Procedure Performance Qualification: Otherwise known as method validation of the final design space against the parameters for intended use of the analytical procedure as defined in the ATP and elucidated during the PPD phase of the life cycle.
3. Procedure Performance Verification: After formal release, ongoing assessment is necessary to monitor the procedure’s performance during operational use, for example, trending data and results over time. Any changes made within the design space are considered validated and can be made without formal change control. Changes outside the design space return to either phase 2 for qualification or, if major, revise the ATP with further development. This is the longest portion of the life cycle and it is imperative that it is controlled and monitored to ensure verification against intended purpose.
The key to a life cycle approach for any analytical procedure is to define the intended purpose as required by the GMP regulations. This is achieved by writing an ATP to provide the foundation for all other work in the life cycle.
Get the ATP wrong and the procedure is not fit for intended purpose.
ICH Catchup: Revision of Q2(R1) and Writing Q14
In 2018, the ICH established an Expert Working Group (EWG) to revise Q2(R1) and write Q14 (20). The aims of the EWG were:
- Q2 required revision, as it lacked guidance for near-infrared spectroscopy (NIR) and Raman spectroscopy, particularly using multivariate models, resulting in inadequate validation reports in regulatory submissions and multiple questions being asked by regulators.
- Development of Q14 as a new guideline outlining the principles and scientific approaches for analytical procedure development
- Specifically, the feasibility of combining both documents into one for simplification and clarity should be examined.
ICH guidances go through a five-step process:
- Step 1: Define the scope and drafting by the Expert Working Group (EWG)
- Step 2: Issue of the draft for public comment (the stage that Q2[R2] and Q14 are at now)
- Step 3: Review of public comments and update of the document
- Step 4: Final approved version released
- Step 5: Incorporation into a member’s national regulatory framework. This can vary from updating regulations to issuing a guidance for industry.
Let us now look at the contents of these two draft guidance documents. Avid readers should also read a review of these two draft documents by Teasdale et al. (21), who have been involved in analytical procedure life cycle development for a number of years.
Two Into One? No Chance!
The first thing to realize is that the EWG have failed to combine both documents to define, simplify, and clarify a unified approach to life cycle management of analytical procedures. This leaves analysts in regulated laboratories juggling two regulatory documents with differing approaches on the same subject. A great start it is not.
This leaves ICH Q2(R2) (22) and its continuing failure to mention the most critical parts of the life cycle: method development and on-going performance verification. Given the pharmaceutical industry’s inability to change, this means perpetuating poor science. Furthermore, the failure to consider a life cycle approach breaks the principles outlined in ICH’s own publications of ICH Q8 and Q12. In summary, the revision of ICH Q2(R2) is a tweak not a substantive advance; the issues mentioned about lack of validation in regulatory submissions are discussed in ICH Q14.
To be fair to the EWG, it is argued that they wanted to maintain the status quo, but this brings with it its own problems. Will analysts read ICH Q14 and Q2(R2) and integrate them together? Probably not. It has not stopped USP <1220>, which has managed to incorporate all phases in a single general chapter (11).
ICH Q2(R2): The Bad Bits
Let’s start with the major flaws in the draft (22):
- Does Q2(R2) define an analytical procedure life cycle? No.
- Does it define an ATP to document intended use of the procedure? No. Q2(R2) does mention “performance characteristics” as the nearest to ATP.
- Method development is still conspicuous by its absence. This is a critical component of the life cycle that has been missing throughout the various versions of Q2.
- There is no mention of validating the analytical procedures against the intended purpose as defined by an ATP. Although Figure 1 shows the linkage between ICH Q2(R2) and Q14, the real linkage is the ATP. But if these documents are to remain separate, they should be linked via procedure of the ATP.
- The only mention of ATP in the whole of the Q2(R2) is in the glossary, which is copied from ICH Q14.
The draft guidance jumps straight into a validation study for the procedure, assuming that development is not done, undocumented, or cobbled together. Given the fact that ICH Q8 and Q12 both focus on life cycle and the proposed ICH Q2(R2) does not, this leaves us with the assumption that the authors are happy to accept a suboptimal and unscientific approach.
ICH Q2(R2): The Good Bits
On the plus side is the expansion of the scope of ICH Q2(R2) to include:
- Spectroscopic methods such as nuclear magnetic resonance (NMR), NIR, and Raman assays for both identification and quantitation.
- Given the large number of biological products and biosimilars that are available and in development, biological assays (polymerase chain reaction [PCR], cell‑based, and binding assays) are included in an expanded ICH Q2(R2) draft. This has resulted in an expansion of calibration methods to accommodate them.
- Section (3.1) covers validation during the life cycle, which was not mentioned in R1. However, it only mentions validation due to changes or transfer, but not as continuous performance verification.
- Good results require a good calibration model and the draft guidance includes calibration models that are linear, nonlinear, and multivariate. Use any calibration method but keep it appropriate—and use the simplest model for your data.
- Linearity as a validation parameter is only applicable now for linear calibration models.
In Annex 1, there is a good process flow diagram leading from the objective of an analytical procedure that then defines which parameters need to be considered during a validation (not forgetting development as well): specificity, range, accuracy, and precision. For example, a procedure for identification would focus on specificity in contrast to an assay where all four would be determined. This is better than the table in the current version of ICH Q2(R1) (10).
ICH Q14: A Life Cycle Approach (Mostly)
In contrast, ICH Q14 (23) aligns with ICH Q8 in terms of a life cycle approach to analytical procedures but with a big gap—validation of the procedure, which is devolved to ICH Q2(R2). Section 2.2 discusses minimal versus enhanced approaches. The use of the term minimal is a concern because it is often interpreted as this is all that needs to be done. Only in the enhanced option is the ATP mentioned (23) and this is defined as:
The ATP defines what is wanted from the procedure but not how it is achieved. However, from the ATP comes the outline of the analytical procedure with knowledge of the analyte and matrix:
- Any sampling and sample storage requirements
- Any sample preparation required
- Instrumental technique used to analyze the sample
- Any data interpretation or calculation required
- Nature of the reportable result.
A good idea is to define the ATP for any analytical procedure, including the minimal approach. However, without an ATP is the minimal approach scientifically sound (3)? No. Outlines of the minimal and enhanced approaches are shown in Table 1 (23). Apart from defining the ATP, the critical component of the enhanced approach is the definition of an analytical control strategy. Here time is taken to identify the parameters that are critical to ensuring a robust analytical procedure and how they must be controlled. Knowing how a component can influence the outcome is essential, such as, for example, what happens to the separation if you have more or less organic modifier or aqueous buffer in the mobile phase.

What is not shown in Table 1 is the iterative nature of the development process between the analytical control strategy and the results from experiments. As more information and knowledge about the procedure
are generated, the procedure and control strategy can be refined.
Risk management is used to identify critical parameters that may need to be carefully controlled and others that have a lesser or zero impact on the outcome of the procedure.
Experimantal design software can be used to devise experiments that will define the procedure design space. Applications can be either standalone or can control instruments via a chromatography data system. Having this information before the validation of the procedure as well as when it is used operationally is essential, as changes within the design space can be made without the need for revalidation.
ICH Q10 Section 10 deals with submission of analytical procedure.
One question that arises is why is this not in ICH Q2(R2)? This is
where the bulk of the data are generated, and is a good argument for merging the two documents.
ICH Grand Canyon of Emptiness
It is often said that a camel is a horse designed by committee and with the two ICH documents we have two camels. Let us explain.

What do we mean by the word life cycle? A reasonable definition would be a series of changes from birth to death. Thus, an analytical procedure life cycle should encompass the beginning of the method through its development, validation, and operation. What is missing from the two ICH documents? The operational phase and monitoring of the validated procedure! This is the critical Grand Canyon of omission by the ICH EWG and the longest part of the life cycle. Table 2 maps three phases of a procedure life cycle for the two
ICH documents and USP <1220>; it is clear that ICH has no consideration for operational monitoring of a procedure.
In addition, we suggest that by this omission the ICH approach fails to comply with the EU GMP Chapter 6 requirements (4):
In conclusion, the ICH EWG must revise these documents to include a full life cycle; alternatively, use the USP <1220> approach for a complete life cycle in a single document.
Summary
Whilst Q2(R2) and Q14 represent significant progress, the lack of consistency between them is disappointing. Had the EWG seen fit to produce a combined document then this would have been avoided. However, the largest issue remains from a life cycle perspective.
Validation is a journey not an event. The USP <1220> has clearly got this journey process right. ICH has only managed to cover stage 1 with Q14 and stage 2 with a revised Q2(R2). Why has ICH ignored the longest and most important life cycle component of performance verification during actual use (stage 3)? ICH should now consider a new guideline to cover this deficiency and complete the validation life cycle process.
Perhaps history will repeat itself and, just as USP<1225> was a precursor to ICH Q2, USP<1220> could provide the stimulus and input to a new ICH guideline for stage 3. We hope so.
Acknowledgements
We would like to thank Jane Weitzel and Margarita Sabater for their review comments.
References
- R.D. McDowall, Data Integrity and Data Governance: Practical Implementation in Regulated Laboratories (Royal Society of Chemistry, Cambridge, UK, 2019).
- R.D. McDowall, LCGC North America 37(1), 44–51 (2019).
- 21 CFR 211, Current Good Manufacturing Practice for Finished Pharmaceutical Products (Food and Drug Administration, Silver Spring, Maryland, USA, 2008).
- EudraLex, Volume 4 Good Manufacturing Practice (GMP) Guidelines, Chapter 6 Quality Control (European Commission, Brussels, Belgium, 2014).
- FDA Guidance for Industry, Submission of Quality Metrics Data, Revision 1 (Food and Drug Administration, Rockville, Maryland, USA, 2016).
- FDA Guidance for Industry, Investigating Out-of-Specification (OOS) Test Results for Pharmaceutical Production (Food and Drug Administration, Silver Spring, Maryland, USA, 2022).
- United States Pharmacopeia General Chapter <1225> “Validation of Compendial Procedures” (United States Pharmacopeial Convention, Rockville, Maryland, USA).
- United States Pharmacopeia General Chapter <1226> “Verification of Compendial Procedures” (United States Pharmacopeial Convention, Rockville, Maryland, USA).
- United States Pharmacopeia General Chapter <1224> “Transfer of Analytical Procedures” (United States Pharmacopeial Convention, Rockville, Maryland, USA).
- International Council for Harmonisation, ICH Q2(R1) Validation of Analytical Procedures: Text and Methodology (International Conference for Harmonisation, Geneva, Switzerland, 2005).
- United States Pharmacopeia General Chapter <1220> “Analytical Procedure Lifecycle” (United States Pharmacopeial Convention, Rockville, Maryland, USA).
- G.P. Martin et al., Pharmacopoeial Forum 38(1)(2012).
- C. Burgess et al., Pharmacopoeial Forum 42(2) (2016).
- E. Kovacs et al., Pharmacopoeial Forum 42(5) (2016).
- K.L. Barnett et al., Pharmacopoeial Forum 42(5) (2016).
- G.P. Martin et al., Pharmacopoeial Forum 43(1), (2017).
- United States Pharmacopeia General Chapter <1220> “Analytical Procedure Life Cycle in process revision” (United States Pharmacopeial Convention, Rockville, Maryland, USA, 46[5], 2020).
- International Council for Harmonisation, ICH Q8: Pharmaceutical Development (International Conference for Harmonisation, Geneva, Switzerland, 2008).
- International Council for Harmonisation, ICH Q12 Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management (International Conference for Harmonisation, Geneva, Switzerland, 2019).
- International Council for Harmonisation, Workplan for ICH Q14: Analytical Procedure Development and Revision of Q2(R1) Analytical Validation (International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), Geneva, Switzerland, 2018).
- A. Teasdale, P.J. Borman, and A.K. Mullen, Organic Process Research & Development 26(4), 1029–1037 (2022).
- International Council for Harmonisation, ICH Q2(R2) Validation of Analytical Procedures, Step 2 Draft (International Conference for Harmonisation, Geneva, Switzerland, 2022).
- International Council for Harmonisation, ICH Q14 Analytical Procedure Development. Step 2 draft (International Conference for Harmonisation, Geneva, Switzerland, 2022).
About The Author
Chris Burgess is Managing Director of Burgess Analytical Consultancy Limited, in Barnard Castle, UK. He is also a member of Pharmaceutical Technology Europe’s editorial advisory board.
About The Column Editor
Bob McDowall is Director of R.D. McDowall Limited, Bromley, UK. He is also a member of LCGC Europe’s editorial advisory board. Direct correspondence to: amatheson@mjhlifesciences.com

What Goes in a CDS IT Service Level Agreement?
Published: April 7th 2025 | Updated: April 7th 2025Protecting your network chromatography data system (CDS) data is critical and a service level agreement (SLA) with your IT provider is vital. What should be included? Are SLAs for in-house IT and SaaS (software as a service) similar?
Rethinking Chromatography Workflows with AI and Machine Learning
April 1st 2025Interest in applying artificial intelligence (AI) and machine learning (ML) to chromatography is greater than ever. In this article, we discuss data-related barriers to accomplishing this goal and how rethinking chromatography data systems can overcome them.
Leveraging an Enterprise Laboratory Informatics Platform to Maximize Scientific Data Advantage
September 9th 2024As data volumes and expectations for fast scientific discovery continue to increase, laboratory-based research organizations can no longer rely on a siloed approach to data management. To remain competitive, scientific organizations need to connect all their data, from discovery through manufacturing, in a unified informatics platform.
Advances in Chromatography Using Artificial Intelligence and Machine Learning
July 3rd 2024Scientists from the University of Turin, Italy have learned how to combine their complementary competencies in analytical chemistry and big data analytics to achieve significant advances in food science and health.