Separation of Tryptophan Oxidized Peptides from Their Native Forms
The Application Notebook
Oxidation of amino acid residues can alter a protein's biological activity, half-life and immunogenicity. Peptide maps can be used to detect oxidized peptide fragments. Peptides are commonly separated by RP-HPLC; however, hydrophobic interaction chromatography (HIC) is an alternative technique offering different selectivity.
Oxidation of amino acid residues can alter a protein's biological activity, half-life and immunogenicity. Peptide maps can be used to detect oxidized peptide fragments. Peptides are commonly separated by RP-HPLC; however, hydrophobic interaction chromatography (HIC) is an alternative technique offering different selectivity.
In this note, we show that native Luteinizing Hormone-Releasing Hormone (LH-RH), a tryptophan (Trp)-containing peptide, is resolved from its forcibly oxidized variants using a ProPac HIC-10 column (Dionex). The separation of oxidized LH-RH revealed four peaks in addition to the two peaks from the native peptide sample. The native and oxidized LH-RH peaks were identified using mass spectroscopy.
Equipment and Methods
A bioinert liquid chromatography system (Dionex ICS-3000 or UltiMate 3000 Titanium system) was used to eliminate metal complex formation or redox reactions with peptides. The LH-RH oxidization is described in Dionex Application Note 211.1
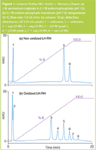
Figure 1
Results and Discussion
Figure 1(a) shows the elution of two non-oxidized LH-RH peaks from the ProPac HIC-10 column, at 11 and 18 min (peaks 5 and 6, respectively). Figure 1(b) shows four additional peaks when the Trp in LH-RH is forcibly oxidized, and the reduced peak areas for peaks 5 and 6 indicate incomplete oxidation. Four different oxidation products, hydroxy-tryptophan (HTRP), N-formylkynurenine (NFK), kynurenine (KYN) and 3-hydroxykynurenine (3OHKYN) have been described by E.L. Finley et al.2 MS analysis of the oxidized LH-RH shows the presence of four additional major ions (data not shown). MS analysis of the non-oxidized LH-RH showed a single major component with a mass-to-charge ratio equal to that expected for LH-RH. The forced oxidation peaks were identified as peptides having one of two Trp oxidation products, hydroxy-tryptophan or N-formylkynurenine (Table 1).
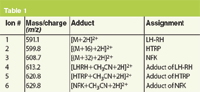
Table 1
Conclusion
The ProPac HIC-10 column can separate oxidized peptides from their native forms showing HIC to be an alternative to RP-HPLC for separation of peptide oxidation variants.
References
1. Dionex Corporation "Hydrophobic Interaction Chromatography for Separation of Tryptophan and Methionine Oxidized Peptides from Their Native Forms," Application Note 211, LPN 2110, Sunnyvale, California, USA. In press, 2009.
2. E.L. Finley et al., Protein Science, 7, 2391–2397 (1998).
ProPac and UltiMate are registered trademarks of Dionex Corporation.
Dionex Corporation
1228 Titan Way, PO Box 3603, Sunnyvale, California 94088, USA
tel. +1 408 737 0700 fax +1 408 730 9403
Website: www.dionex.com

Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
University of Oklahoma and UC Davis Researchers Probe Lipidomic Profiles with RP-LC–HRMS/MS
May 6th 2025A joint study between the University of Oklahoma Health Sciences Center (Oklahoma City, Oklahoma) and the UC Davis West Coast Metabolomics Center (Davis, California) identified differentially regulated lipids in type 2 diabetes (T2D) and obesity through the application of reversed-phase liquid chromatography-accurate mass tandem mass spectrometry (RP-LC-accurate MS/MS).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)

















