Analysis of 17-Pesticide Residues in Apple Using Agilent SampliQ QuEChERS EN Kits and Agilent J&W Ultra Inert columns by GC–MS
The Application Notebook
The QuEChERS method for pesticide analysis was first introduced by USDA scientists in 2003.1 The EN method 15662:2007 is a European variation to the QuEChERS method.2,3
Introduction
The QuEChERS method for pesticide analysis was first introduced by USDA scientists in 2003.1 The EN method 15662:2007 is a European variation to the QuEChERS method.2,3 The method uses acetonitrile extraction, followed by the salting out of water from the sample using anhydrous magnesium sulphate (MgSO4), NaCl and buffering citrate salts to induce liquid–liquid partitioning. A dispersive solid-phase extraction (dispersive SPE) is conducted for clean-up using a combination of primary secondary amine (PSA) to remove fatty acids among other components and anhydrous MgSO4 to reduce the remaining water in the extract. After mixing and centrifugation, the upper layer is ready for analysis. Gas chromatography–mass spectrometry (GC–MS) has been widely used in pesticide analysis for many years. Many pesticides are volatile or semi-volatile, which makes them GC-amenable compounds.
Experimental
Materials:
- Agilent SampliQ QuEChERS EN extraction kit, p/n 5982-5650
- Agilent SampliQ QuEChERS EN dispersive SPE kits for general fruits and vegetables, p/n 5982-5021 and 5982-5056
Instrument condition:
An Agilent GC–MS method for pesticides analysis was used for this study.4
Results and Discussion
Agilent's SampliQ extraction salts are uniquely prepared in an anhydrous package. The addition of a food sample with a high content of water directly to the salts creates an exothermic reaction, which can affect analyte recoveries, especially for volatile pesticides. The unique SampliQ anhydrous salts packet allows salt addition AFTER the addition of organic solvent to the sample, as specified in the original QuEChERS methodology. Figure 1 shows the flow chart for the QuEChERS EN sample extraction procedure.

Figure 1: Flow chart of the Agilent SampliQ QuEChERS EN extraction procedure.
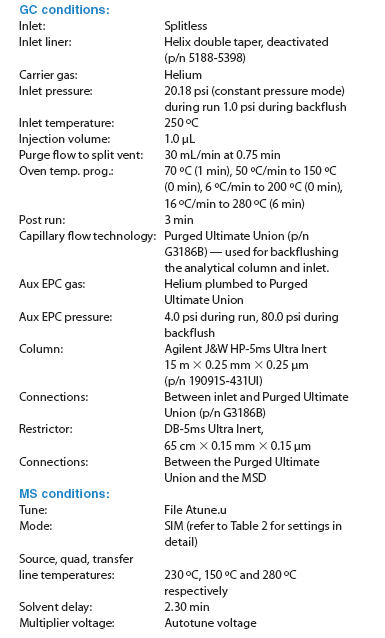
QuEChERS food samples still usually contain high-boiling indigenous impurities, which can accumulate on the head of the column, causing peak tailing and retention time shift. Over time, these impurities can migrate to the mass spectrometer (MS) source, causing contamination of the source. Therefore, column backflushing was employed to increase column life as well as preserve the MS source. Agilent's capillary flow technology makes column backflushing routine.4–6 In this study, the GC–MS system used a Purged Ultimate Union. The analytical column was connected to the capillary flow device. A short restrictor (65 cm × 0.15 mm × 0.15 μm of DB-5ms Ultra Inert column) was used to couple the capillary flow device to the mass spectrometer. Figures 2(a) and (b) show the chromatograms of a blank apple extract and a 50 ng/g fortified apple extract.
Figure 2
Recovery and reproducibility: The recovery and reproducibility were evaluated by spiking pesticides standards in comminuted apple sample at levels of 10, 50 and 200 ng/g. These QC samples were quantified against the matrix spiked calibration curve. The analysis was performed in replicates of six (n = 6) at each level. It can be seen from the results that all of the pesticides give good recoveries (average of 84.7% for 1 mL and 87.2% for 6 mL) and precision (average of 4.3% RSD for 1 mL and 5.1% RSD for 6 mL). Figure 3 shows the recovery and precision results for 1 mL dispersive SPE and 6 mL dispersive SPE.
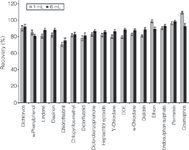
Figure 3: The recovery and precision results of 1 and 6 mL sample volumes employing Agilent SampliQ Dispersive SPE, 2 and 15 mL kits, respectively.
Conclusions
Agilent's SampliQ QuEChERS EN extraction and dispersive SPE kits for general fruits and vegetables provide a simple, fast and effective method for the purification and enrichment of representative volatile to semi-volatile pesticides in apple. The LOQs of the pesticides were lower than regulated MRLs in apple. Because the selected pesticides represented a broad variety of different classes and properties, the Agilent SampliQ QuEChERS EN extraction and dispersive SPE kits for general fruits and vegetables is an excellent choice for other pesticides in similar food matricies.
References
1. M. Anastassiades and S.J. Lehotay, J. AOAC Int., 86, 412–431 (2003).
2. European Committee for Standardization/Technical Committee CEN/TC 275 (2007), Foods of plant origin: Determination of pesticide residues using GC-MS and/or LC-MS/MS following acetonitrile extraction/partitioning and cleanup by dispersive SPE QuEChERS method. European Committee for Standardization, Brussels
3. P. Payá and M. Anastassiades, Anal. Bioanal. Chem., 389, 1697–1714 (2007).
4. P.L. Wylie and C.K. Meng, "A Method for the Trace Analysis of 175 Pesticides Using the Agilent Triple Quadrupole GC/MS/MS," Agilent Technologies publication 5990-3578EN.
5. C.K. Meng, "Improving Productivity and Extending Column Life with Backflush," Agilent Technologies publication 5989-6018EN.
6. P.L. Wylie, "Direct Injection of Fish Oil for the GC-ECD Analysis of PCBs: Results Using a Deans Switch with Backflushing," Agilent Technologies publication, 5989-6095EN.

Agilent Technologies Inc.
2850 Centerville Road, Wilmington, Delaware 19808, USA
tel. +1 800 227 9770 fax +1 302 633 8901
E-mail: info_agilent@agilent.com Website: www.agilent.com/chem

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
Investigating the Protective Effects of Frankincense Oil on Wound Healing with GC–MS
April 2nd 2025Frankincense essential oil is known for its anti-inflammatory, antioxidant, and therapeutic properties. A recent study investigated the protective effects of the oil in an excision wound model in rats, focusing on oxidative stress reduction, inflammatory cytokine modulation, and caspase-3 regulation; chemical composition of the oil was analyzed using gas chromatography–mass spectrometry (GC–MS).


















