Trace-Level Aliphatic Amines in Cationic Drugs
The Application Notebook
The analytical challenge treated in the present work consists in determining sub-ppb concentrations of low-molecular-weight amines in the presence of strongly retained cationic drugs by using ion chromatography (IC) with upstream in-line coupled-column matrix elimination (CCME).
The analytical challenge treated in the present work consists in determining sub-ppb concentrations of low-molecular-weight amines in the presence of strongly retained cationic drugs by using ion chromatography (IC) with upstream in-line coupled-column matrix elimination (CCME). In contrast to direct-injection IC, where the late elution of strongly retained drugs is a significant drawback and requires eluents with added acetonitrile, the low-pressure CCME technique — by using two preconcentration columns in series — gets by without solvent-containing eluents. In an "inverse matrix elimination" step, cationic drug and target amines are trapped on a high-capacity and a very-high-capacity preconcentration column, respectively. During the separation step, a rinsing solution flushes the drug retained on the high-capacity preconcentration column to waste.
Application of CCME significantly shortens the analysis time, protects the analytical column and improves sensitivity, as well as selectivity. Besides the determination of monomethylamine in Nebivolol hydrochloride discussed here, the CCME technique is a promising tool for determining further low-molecular-weight amines in a wide range of drugs.
Low-molecular-weight amines find widespread use in raw materials or intermediate products, in the manufacturing of numerous chemicals, pharmaceuticals, polymers, pesticides, rubber, dyes, adhesives, solvents and corrosion inhibitors. Their monitoring is crucial as most of them are toxic. Their determination, however, is a challenging task because the protonated amines are often poorly retained on the column, which results in very short retention times, poor separation and strongly asymmetric peaks. Separations become even more demanding in the presence of interfering cationic drug ingredients.
These shortcomings can be overcome by applying coupled-column matrix elimination (CCME). After optimization of the CCME parameters, trace levels of monomethylamine (MMA) are determined in the cationic beta blocker Nebivolol hydrochloride. Alternatively, direct-injection results are presented.
Experimental
Instrumentation
Upstream matrix elimination uses a high-capacity (Metrosep C PCC 1 HC) and a very-high-capacity preconcentration column (Metrosep C PCC 1 VHC) in series. Three peristaltic pumps PP (Figure 1) take over the complete sample transfer steps within the CCME. All determinations were performed on the 850 Professional IC – Cation – Prep 2. Chromatographic conditions are indicated in the respective chromatograms. All columns and instruments are from Metrohm.
Amine standards, drug samples and eluents
All reagents used in this work were of the highest purity grade (puriss. p.a.). Eluents were prepared with deionized water having a specific resistance higher than 18 MΩ·cm. While suprapure nitric and hydrochloric acid for eluent preparation were obtained from Merck Germany, analytical grade acetonitrile was purchased from Merck India. Amines or their respective hydrochloride salts were purchased from Sigma-Aldrich Switzerland (Fluka brand). Nebivolol hydrochloride samples originated from Heterolab (Hyderabad, India).
Results and Discussion
Coupled-column matrix elimination
a) The sample is transferred to the Metrosep C PCC 1 HC high-capacity preconcentration column, which traps the amines and the cationic drug [Figure 1(a)]. Sample transfer occurs by a peristaltic pump using ultrapure water that was previously freed of trace cations by passing through a high-capacity trap column.
b) While the cationic drug is retained on the Metrosep C PCC 1 HC, amines are selectively eluted with eluent E2 and subsequently trapped on the Metrosep C PCC 1 VHC very-high-capacity preconcentration column [Figure 1(b)]. The optimization of eluent composition E2 and corresponding transfer time is described in the next chapter.
c) The trapped amines are eluted with eluent E1 and transferred to the separation column [Figure 1(c)]. Simultaneously, the rinsing solution R1, having a high organic modifier content, flushes the drug from the cation-retaining Metrosep C PCC 1 HC to waste. The Metrosep C PCC 1 HC preconcentration column is now ready for the next sample.
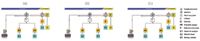
Figure 1: Schematic illustration of the low-pressure coupled-column matrix elimination.
Optimization of eluent composition E2 and transfer time
By means of the early-eluting monomethylamine (MMA) and dimethylamine (DMA), eluent composition E2 and the corresponding transfer time (flow-rate = 0.47 mL/min) were optimized in terms of amine recovery. The latter was calculated by comparing the peak area obtained after matrix elimination with the peak area after direct injection onto the C PCC 1 VHC preconcentration column. According to Figure 2, best results for MMA and DMA determination were obtained for 1.0 mmol/L nitric acid as eluent E2 and a transfer time of 0.35 min.

Figure 2: Optimization of eluent composition E2 and transfer time for (a) MMA and (b) DMA.
MMA in Nebivolol hydrochloride
By means of MMA determination in Nebivolol hydrochloride — an exemplary cationic drug and cardioselective beta blocker with pronounced antihypertensive effect — the performance of the common direct-injection procedure and of the previously described CCME are illustrated.
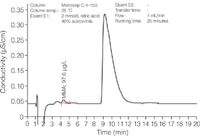
Figure 3: Direct-injection chromatogram of Nebivolol hydrochloride spiked with 100 μg/L MMA.
a) Direct injection
Acetonitrile additions to the eluent reduce the strong lipophilic interactions of the cationic pharmaceutical with the column's carrier material and thus shorten the drug's retention time. However, such organic solvent additions impair detection sensitivity and selectivity. While direct injection is still feasible for MMA determination in Nebivolol hydrochloride (Figure 3), monitoring of higher amine homologues in strongly retained cationic drugs becomes increasingly difficult.
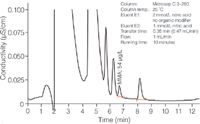
Figure 4: Chromatogram of Nebivolol hydrochloride spiked with 50 μg/L MMA after upstream CCME.
b) CCME
CCME allows for a rapid determination of trace-level MMA with recoveries ranging between 101 and 106% (Table 1). Relative standard deviations (RSDs) are better than 5%. The method excels by its capability to simultaneously determine various low-molecular-weight amines in strongly retained cationic drugs.

Table 1: MMA recoveries in Nebivolol hydrochloride samples.
References
1. N. Harihara Subramanian, "Development of novel methods for the determination of trace ionic contaminants in complex matrices using ion chromatography," PhD thesis, University of Madras, Chennai, India, in preparation (2009).
2. Metrohm Application Notes AN-C-007, AN-C-052, AN-C-057, ANC-070; AN-C-078, AN-C-092...AN-C-093 and AN-C-124... AN-C-126 (downloadable under http://products.metrohm.com).

Metrohm International Headquarters
CH-9101 Herisau, Switzerland
tel. +41 71 353 85 04
E-mail: aw@metrohm.com
Website: www.metrohm.com

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.



















