Fast Analysis of Charge Variants of Monoclonal Antibodies by Ion Exchange Chromatography
The Application Notebook
Development and production of biopharmaceuticals is a growing segment of the pharmaceutical industry. The development of vaccines is only one - but as a result of the current H1N1 flu hysteria it is the most striking - segment of this emerging field. Recombinant proteins, ranging from insulin products to therapeutic antibodies, represent another important group of biopharmaceuticals. With the introduction of the first so-called biosimilars in Europe the demand for highly efficient analysis methods is further increasing. Newly developed stationary phases for the typical modes of biochromatography of native proteins such as SEC or IEC open up new opportunities for increasing throughput in the bioanalytical lab.
Development and production of biopharmaceuticals is a growing segment of the pharmaceutical industry. The development of vaccines is only one — but as a result of the current H1N1 flu hysteria it is the most striking — segment of this emerging field. Recombinant proteins, ranging from insulin products to therapeutic antibodies, represent another important group of biopharmaceuticals. With the introduction of the first so-called biosimilars in Europe the demand for highly efficient analysis methods is further increasing. Newly developed stationary phases for the typical modes of biochromatography of native proteins such as SEC or IEC open up new opportunities for increasing throughput in the bioanalytical lab.
Introduction
Biopolymers are highly complex, large molecules requesting powerful analysis methods for a proper characterization. Most proteins are expressed in several isoforms differing by modifications of individual amino acid side chains, or the N- or C-terminus. Typical modifications are deamidation, phosphorylation, acetylation, methylation, oxidation or glycosilation.
Isoforms may differ in biological activity and stability, which is critical when producing protein therapeutics. Therefore, a thorough characterization and quantification of the isoforms is inevitable to guarantee a consistent product quality. Rapid, reliable and quantitative analytical methods are needed. Thus, chromatographic and electrophoretic methods are the preferred analytical tools for the analysis of micro heterogeneity of recombinant proteins.1 Chromatographic methods are easy to automate, more robust and reproducible and less influenced by the sample matrix than electrophoretic techniques.
For quality control (QC) of biopharmaceuticals, chromatographic methods are preferably used. Ion exchange- (IEC) and size exclusion chromatography (SEC) are the methods of choice for the separation of proteins in native form. Typical analysis times in biochromatography are 30 minutes or even in the range of hours. Reducing particle sizes and optimizing HPLC systems would be the first idea to increase separation efficiency and speed up the analysis, similar to current trends in the separation of small molecules. This was already realized by introducing 2.5 μm non-porous IEC phases or 4.0 μm SEC phases some years ago. Unfortunately, the large size of some analytes and difficult matrices limit the use of very small particles in biochromatography.
Innovative techniques for surface modification, which were already successfully applied to the latest generation of ion exchange process resins by Tosoh Bioscience, were now used to develop new ion exchange stationary phases for the HPLC of biopolymers. Based on their high separation efficiency, the new stationary phases allow both, enhancing resolution and peak capacity or shortening analysis time.
New Stationary Phases
Available in three different chemistries, TSK-GEL STAT columns are packed with mono-disperse non-porous particles of which the surface consists of an open access network of multilayered ion-exchange groups (Figure 1). The innovative bonding chemistry combined with a relatively large particle size results in a respectable loading capacity and a low operating pressure. The non-porous, hydrophilic base material of the packing provides a fast mass transport — the prerequisite for high performance at reduced analysis time. Various column formats and particle sizes are available to match specific application needs: Short columns packed with 10 micron particles are ideally suited for fast and ultrafast analysis. High resolution separations are achieved by using longer columns packed with smaller beads.
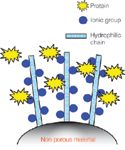
Figure 1: Particle Design of TSK-GEL STAT Columns.
Analysis of MAb Charge Variants
Cation exchange chromatography is a common method for QC analysis of therapeutic proteins. It is often used to separate and quantify charge isoforms of proteins resulting from deamidation of asparagine or glutamine residues or from incomplete removal of C-terminal lysine residues.2,3 To show the potential of the newly developed IEC columns for increasing throughput in QC of biopharmaceuticals we applied TSKgel CM-STAT cation exchange columns to separate charge variants of several monoclonal antibodies.
Results
The typical analysis time of about 40 minutes could be significantly reduced when separation was performed on a 10 cm CM-STAT column, filled with 7 μm particles of the weak cation exchanger. The analysis profiles for five antibodies separated on the TSKgel CM-STAT column were compared with the profiles obtained on a competitive, pellicular 25 cm long WCX column, which is most often used for this type of analysis (Figure 2). Similar or higher (see mAb A) resolution profiles were obtained on TSKgel CM-STAT in approximately half the time.

Figure 2: Reduction of analysis time for the separation of charge variants of monoclonal antibodies on TSKgel CM-STAT (a) compared to the separation on a competitive WCX column (b).
Conclusion
Latest innovations in surface chemistry and particle design were the key factors for developing a new generation of ion exchange columns. As shown above, the high resolution type of TSK-GEL STAT columns can significantly improve current QC methods for the analysis of antibody variants. The high throughput types of TSK-GEL STAT columns are ideally suited for fast analysis for process control of screening purposes. For example the monitoring of protein PEGylation reactions in intervals of a few minutes.
TSK-GEL STAT columns support biochemists in increasing lab productivity by establishing high throughput HPLC analysis not only for small organic molecules but also for large biopolymers.
References
1. K. Ahrer and A. Jungbauer, J. Chromatogr. B, 841, 110–122 (2006).
2. R.J. Harris et al., J. Chromatogr. B, 752, 233–245 (2001).
3. M. Weitzhandler et al., J. Chromatogr. A, 828, 364–372 (1998).

Tosoh Bioscience GmbH
Zettachring 6, 70567 Stuttgart, Germany
tel. +49 711 13257 0 fax +49 711 13257 89
E-mail: info.sep.eu@tosoh.com
Website: www.tosohbioscience.com

A Novel LC–QTOF-MS DIA Method for Pesticide Quantification and Screening in Agricultural Waters
May 8th 2025Scientists from the University of Santiago de Compostela developed a liquid chromatography quadrupole time-of-flight mass spectrometry (LC–QTOF-MS) operated in data-independent acquisition (DIA) mode for pesticide quantification in agriculturally impacted waters.
Distinguishing Alcohol- from Non-Alcohol-Associated Liver Cirrhosis with LC-MS
May 7th 2025A pilot study investigating whether nicotinamide adenine dinucleotide kinase (NADK) expression is selectively diminished in alcohol-associated liver cirrhosis (AC), as well as evaluating its potential as a biomarker for this condition, measured AC and non-AC (NAC). Nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADP+) levels in human liver samples were measured using liquid chromatography-mass spectrometry (LC-MS).
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)

















