High Resolution Peptide Mapping of Ig-G Using Kinetex C18
The Application Notebook
A critical part of research and quality control analysis of proteins involves verifying the primary and secondary structure of a protein. Peptide mapping is the main technique used to determine the structure of a protein as well as identify any post-translational modifications.
Introduction
A critical part of research and quality control analysis of proteins involves verifying the primary and secondary structure of a protein. Peptide mapping is the main technique used to determine the structure of a protein as well as identify any post-translational modifications. Such analyses are critical for Ig-G based therapeutics; as such, modifications can critically affect the immunogenicity and activity of a protein. For Ig-G such analyses are especially difficult because the large number of peptides generated from an enzymatic digestion make it difficult to fully resolve all peptides by reversed-phase HPLC. Key to the best peptide maps is using a HPLC column that generates the maximum resolution and efficiency. Kinetex C18 is a recently introduced solid core HPLC column that generates efficiencies higher than many leading UHPLC and superficially porous columns. Peptide maps for Ig-G were used as a comparison between Kinetex and traditional C18 columns to demonstrate the performance of this new technology media.
Materials and Methods
HPLC solvents, mobile phase modifiers and Asp-N endoproteinase were obtained from EMD (San Diego, California, USA). Lys-C endoproteinase was obtained from Wako Chemicals (San Diego, California, USA). Human Ig-G2 was obtained from Dako (Carpinteria, California, USA). A Kinetex 2.6 μm C18 column (150 × 4.6 mm) and a wide-pore 3 μm C18 were used for HPLC analysis (Phenomenex, Torrance, California, USA). Human Ig-G was digested with Lys-C for 18 hours at 37 °C (E/S 1:30) in 4 M guanidine/0.1 M ammonium bicarbonate. Sample was diluted to 1 M guanidine then digested with Asp-N for 12 hours (E/S 1:50). The reaction was quenched with TFA and 20 μg aliquots were injected on HPLC. HPLC analysis were performed on an Agilent 1100 HPLC equipped with an autosampler, column oven, and MWD using ChemStation software (Agilent, Santa Clara, California, USA). The mobile phases used were: A was 0.1% TFA in water/2% acetonitrile and B was 0.085% TFA in acetonitrile with a gradient from 2 to 45% B in 30 minutes being used. A flow-rate of 1 mL/min was used and peptide elution was monitored at 214 nm.
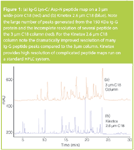
Figure 1
Results and Conclusions
Figure 1 shows the Lys-C/Asp-N peptide map run on a wide-pore 3 μm C18 column (red) compared to the Kinetex 2.6 μm C18 column (blue) with a time offset to match maps. The combination of enzymes is widely used in the industry to digest human Ig-G therapeutics as tryptic digests fail to fully digest the non-reduced protein (data not shown). The data clearly shows a dramatic improvement in peak efficiency as well as number of peptide peaks resolved in the Kinetex 2.6 μm C18 peptide map when compared to an industry standard 3 μm C18 column. Such results show the utility of using the Kinetex column for improved peptide mapping even without optimized flow-rates. However, unlike other high efficiency solutions that require a UHPLC to run a sub-2 μm column, the high resolution Kinetex peptide map was run on a standard HPLC system at backpressure of around 280 bar in this application. This data resoundingly shows that Kinetex 2.6 μm HPLC columns can deliver high resolution peptide maps for all HPLC systems. Although not shown here, Kinetex 1.7 μm C18 columns can deliver even higher performance if one wishes to use a UHPLC system for their peptide map analysis.
Kinetex is a trademark of Phenomenex Inc.

Phenomenex Inc.
411 Madrid Avenue, Torrance, California 90501, USA
tel. +1 310 212 0555 fax +1 310 328 7768
E-mail: info@phenomenex.com
Website: www.phenomenex.com

Determining the Effects of ‘Quantitative Marinating’ on Crayfish Meat with HS-GC-IMS
April 30th 2025A novel method called quantitative marinating (QM) was developed to reduce industrial waste during the processing of crayfish meat, with the taste, flavor, and aroma of crayfish meat processed by various techniques investigated. Headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) was used to determine volatile compounds of meat examined.
Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)


















