Simultaneous Determination of Mineral Acids, Fluoride, and Silicate in Etching Baths by Ion Chromatography with Dual Detection
The presented ion chromatographic method is used for the simultaneous determination of HF, HNO3, H2SO4, short-chain organic acids and H2SiF6 in acidic texturing baths that are used in the wet chemical etching process of solar cell production.
The presented ion chromatographic method is used for the simultaneous determination of HF, HNO3, H2SO4, short-chain organic acids and H2SiF6 in acidic texturing baths that are used in the wet chemical etching process of solar cell production. Fluoride, nitrate, sulfate, and acetate are determined by conductivity detection after chemical suppression, while the silicon present in the form of hexafluorosilicate is detected spectrophotometrically as molybdosilicic acid after derivatization in the same analysis. The analytical results are validated by titration.
E nergy production from renewable sources such as biomass, biogas, biofuels, water, wind, and solar power is becoming increasingly important in our energy-hungry society. Particular interest is given to solar energy, which by human criteria is inexhaustible. Solar cells used in photovoltaic units convert the radiation energy in sunlight directly into electric energy.
Solar cells are manufactured from ultrapure mono- or polycrystalline silicon wafers whose surface is treated in acid etching baths (also known as texturing baths) before being spiked with foreign atoms (P, B). The etching solutions consist of various acids, which act as an oxidizing agent (HNO3), complexing agent (HF), stabilizer and wetting agent (CH3COOH), or buffers (H3PO4, CH3COOH) and determine the surface structure and thus the efficiency of the solar cells. The replenishment of components used up in the etching process extends the bath life and saves costs, though it does require knowledge of the exact composition of the bath, especially the concentration of silicon and hexafluorosilicate. By using titration and ion chromatography (IC), it is possible to determine the key components quickly and precisely.
This article describes an ion chromatographic method that separates all relevant components in the bath on an anion-exchange column and identifies them by dual detection in a single run. After suppressed conductivity detection of the acid anions, the undissociated silicic acid reacts in a post-column reaction (PCR) to form molybdosilicic acid, which is determined spectrophotometrically at 410 nm. The concentrations of fluoride and hexafluorosilicate are determined by way of a simple stoichiometric calculation that is performed by the chromatography software.
Instruments and Reagents
a) Instrument setup
- 850 Professional IC Anion – MCS with post-column reactor
- 858 Professional Sample Processor
- Lambda 1010 UV/VIS Detector
- 771 IC Compact Interface
- MagIC Net chromatography software
b) Reagents and eluent
The standard solutions were prepared with CertiPUR standards from Merck (SiO2 in NaOH; solutions of NaF and NaNO3 in ultrapure water) and the TraceCERT standard from Fluka (acetate solution). All the standard and eluent solutions were prepared with ultrapure water with a specific resistance of more than 18 M?·cm. Etching bath samples were provided by a solar cell manufacturer from Germany.

Figure 1: 850 Professional IC Anion â MCS and 858 Professional Sample Processor.
Etching of Silicon
In the wet chemical etching of silicon surfaces, nitric acid is used to oxidize silicon to form silicon dioxide which is further etched by hydrofluoric acid.
3 Si + 4 HNO3 + 18 HF —> 3 H2SiF6 + 4 NO + 8 H2O
During the etching process, the concentrations of HF and HNO3 in the etching bath decrease and the concentrations of water and hexafluorosilicate increase. To ensure a constant etching rate and surface properties, the etching bath can be regenerated a few times by subsequent replenishment of spent acids. The increasing concentration of H2SiF6, however, limits the number of possible recycling cycles. This requires semi-continuous monitoring of the bath components, which can be achieved conveniently by automated ion chromatography.
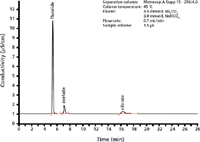
Figure 2: Conductivity chromatogram of a simulated etching bath with 25 mg/L fluoride, 20 mg/L acetate, and 10 mg/L nitrate. The undissociated orthosilicic acid is not recorded in the conductivity detector.
Dual Detection
The acid anions present in the etching bath — mainly fluoride and nitrate, though sometimes also sulfate and acetate — are separated under alkaline elution conditions and determined by conductivity detection (Figure 2). In the alkaline eluent, the hexafluorosilicate is converted into orthosilicic acid which is undissociated and therefore "invisible" in the conductivity detector.
Na2SiF6 + 4 NaOH —> Si(OH)4 + 6 NaF
The undissociated orthosilicic acid is determined by way of post-column reaction with an acid molybdate solution and subsequent UV-vis detection at 410 nm (Figure 3).
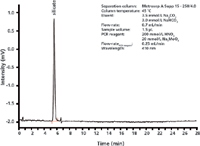
Figure 3: UVâvis chromatogram of a 10 mg/L silicic acid standard. Silicic acid is derivatized to molybdosilicic acid which is then detected spectrophotometrically.
H4SiO4 + 12 MoO42- + 24 H+ —> H4 [Si(Mo3O10)4] + 12 H2O
The injection of SiF62- produces a fluoride peak in the conductivity detector and a silicate peak in the UV–vis detector. The mass balance derived from the respective peak areas confirms that the concentration of SiF62- results stoichiometrically from the determined fluoride and silicate concentrations, provided there are no other sources of fluoride or silicate present. Thus the concentration of free HF can be calculated as the difference between the total fluoride concentration and the fluoride concentration from the hexafluorosilicate:
[HF] = [F- ]total - [F- ]hexafluorosilicate
Analysis of the Texturing Baths and Validation
After being diluted at ratios between 1:1000 and 1:5000, four samples from different texturing baths are analysed for their constituents by using the above IC method with dual detection. Figure 4 shows the chromatograms of etching bath sample 1 obtained by (a) conductivity and (b) UV–vis detection.
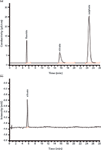
Figure 4: (a) Conductivity and (b) UVâvis chromatograms of etching bath sample 1 with 1:2000 dilution. The chromatographic parameters are the same as those of the prior chromatograms.
Table I provides an overview of the concentrations of the relevant bath components determined by ion chromatography with dual detection. For comparison, the concentrations obtained by titration are also shown. The potentiometric determination of acid concentrations and H2SiF6 content was carried out using aqueous acid–base titration with 1 mol/L NaOH solution.

Table 1: Comparison of the concentrations of a few selected bath components determined by ion chromatography and titration
Conclusion
Ion chromatography with dual detection allows determining the concentration of all relevant constituents of texturing baths for solar cell production in less than 30 min. The acids consumed in the texturing process can subsequently be replenished in a targeted way. This extends the life of the etching baths, guarantees clean and reproducible wafer surfaces, cuts costs, and protects the environment.
References
(1) A. Henssge and J. Acker, Talanta 73, 220–226 (2007).
(2) J. Acker and A. Henssge, Talanta 72, 1540–1545 (2007).
(3) M. Zimmer et al., 22nd European Photovoltaic Solar Energy Conference and Exhibition, Milan, Italy (2007).
(4) G. Bogenschütz, C. Wilde, and C. Hack, Pittcon 2009 (http://www.metrohm.com/com/Applications, search for 8.000.6041EN).
Metrohm International Headquarters
Ionenstrasse, CH-9101 Herisau, Switzerland
tel. +41 71 353 85 04
E-mail: aw@metrohm.com
Website: www.metrohm.com





















