Improving SFC Chiral Separations by Employing a New Chlorinated Polysaccharide Chiral Stationary Phase: The RegisPack CLA-1
The Application Notebook
As the number of chiral columns and chiral separations has grown, it has become difficult to organize the information for use in developing new methods. The success of chiral method development depends on the introduction of appropriate columns. The RegisPack? polysaccharide-based chiral stationary phase (CSP) is most successful in achieving a majority of chiral separations. However, there are cases in which this proven chiral selector leads to partial or no separation. Excellent separations using the newly introduced RegisPack? CLA-1 CSP have been obtained for a range of compounds, either by improving or complementing RegisPack's selectivity.
As the number of chiral columns and chiral separations has grown, it has become difficult to organize the information for use in developing new methods. The success of chiral method development depends on the introduction of appropriate columns. The RegisPack® polysaccharide-based chiral stationary phase (CSP) is most successful in achieving a majority of chiral separations. However, there are cases in which this proven chiral selector leads to partial or no separation. Excellent separations using the newly introduced RegisPack® CLA-1 CSP have been obtained for a range of compounds, either by improving or complementing RegisPack's selectivity.
This study used the well-established RegisPack® CSP and the new chlorinated RegisPack® CLA-1 CSP. Both phases were based on 5-µm silica. Columns were both 25 cm x 4.6 mm in size.
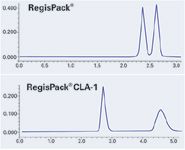
Figure 1: Chromatogram Set 1. Sample: Propafenone. Mobile Phase: CO2/IPA (70/30) + 0.5% DEA. Flow Rate: 4.0 mL/min. Temp: 40 °C. Pressure: 125 bar. UV: 254 nm.
The RegisPack® and RegisPack® CLA-1 are coated with tris-(3,5-dimethylphenyl) carbamate of amylose and tris-(5-chloro-2-methylphenyl) carbamate of amylose, respectively.
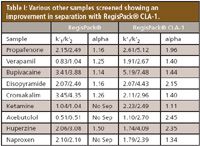
Table I: Various other samples screened showing an improvement in separation with RegisPack® CLA-1.
This study investigated several compounds that had poor or no separation on a RegisPack® column. They were screened on the new RegisPack® CLA-1 column. Following are some chromatographic illustrations and a tabular summary of our results.
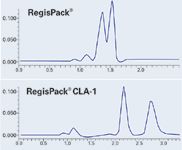
Figure 2: Chromatogram Set 2. Sample: Verapamil. Mobile Phase: CO2/IPA (75/25) + 0.5% DEA. Flow Rate: 4.0 mL/min. Temp: 40 °C. Pressure: 125 bar. UV: 290 nm.
Conclusion
The RegisPack® is a well-established CSP that does a superior job of separating the majority of compounds submitted for chiral screening. There are cases where the RegisPack® CSP can only achieve partial or no separation on some analytes. The new RegisPack® CLA-1 can exhibit unique selectivity in separation when existing CSP's fail. This CSP is highly recommended to be added to the screening process.
Note
All work was performed on a WATERS THAR SFC Method Station.
Regis Technologies, Inc.
8210 Austin Ave, Morton Grove, IL 60053
tel. (847) 583-7661, fax (847) 967-1214
E-mail: teds@registech.com, Website: www.registech.com/chiral





















