Analysis of Pharmaceuticals in Whole Blood by Poroshell 120, Using a Modified Mini-QuEChERS Approach for Sample Preparation
The Application Notebook
Determination of pharmaceuticals in biological matrices is commonly employed in ADME (DMPK), clinical, and forensic analysis. The main techniques used for drug monitoring and analysis are immunoassays, LC, and GC methods.
Determination of pharmaceuticals in biological matrices is commonly employed in ADME (DMPK), clinical, and forensic analysis. The main techniques used for drug monitoring and analysis are immunoassays, LC, and GC methods. Mass spectral chromatographic methods are the first choice for many applications based on their flexibility, selectivity, sensitivity, qualitative, and quantitative capabilities. Analysis of pharmaceuticals in biological samples requires sample preparation that can range from simple protein precipitation (PPT) to more complex solid-phase extraction (SPE). There is a need in classic sample preparation for an approach to determine multi-classes of pharmaceuticals in biological samples. Polymeric or mixed-mode SPE sorbents which can isolate acidic, neutral, and basic drugs by hydrophobic or ion-exchange interactions have addressed this void but there is always room for sample preparation techniques that are rapid and inexpensive to implement.
In 2003, Anastassiades and colleagues. reported a method for the analysis of multiresidue pesticides in foods known as QuEChERS; which equates to a quick, easy, cheap, effective, rugged, and safe sample preparation approach (1). The authors reported outstanding recoveries for a wide range of pesticide classes (1). Since its inception there have been many reported articles employing QuEChERS for the analysis of a wide range of compounds including, but not specific to: antibiotics (2), toxins (3), contaminants (4), and pharmaceuticals (5).
Experimental
In this application note we describe an extension of the work presented by Plössl and colleagues in 2006 (5), for the determination of pharmaceuticals in whole blood resulting in a modified mini-QuEChERS procedure with LC–MS-MS analysis. The experiments used whole blood from human donors and evaluated both nonbuffered and buffered extraction salts of the QuEChERS method, namely the Original, AOAC 2007.01 and EN 15662. Modifications to the acetonitrile (extraction solvent) used in the first step (extraction/partitioning) were also evaluated (Table I). The experiments were performed using nine different pharmaceuticals (lidocaine, tramadol, amitriptyline, biperidene, oxazepam, lorazepam, chlorpromazine, diltiazem, and naloxone) which offer a broad range of hydrophobicity and dissociation constants, pKa.
The general procedure is as follows. A 1 mL aliquot of whole blood was added to a centrifuge tube and spiked with appropriate volume from a concentrated stock mixture to yield 25, 50, and 100 ng/mL of the component mix, add 20 uL of IS stock solution (nortriptyline), two ceramic homogenizers then vortex. Then 2 mL of acetonitrile solutions (with or without acid) was added and vortexed. A premixed amount (see Table I) of the extraction salts is added and vigorously shaken, centrifuged at 5000 rpm for 5 min. One milliliter of the extract is transferred into a d-SPE tube (2 mL centrifuge tube) containing 50 mg of PSA and 100 mg of MgSO4 for matrix clean-up; vortex for 1 min and centrifuge at 18,000 rpm for 3 min. A 200 μL aliquot of the extract is transferred into a LC vial containing 800 uL of water, vortexed and analyzed. A matrix matched calibration curve from 10–250 ng/mL was employed to determine recovery.
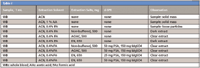
Table I
Results
The experiments showed that the use of ACN (0.4% FA) as the extraction solvent offered a better lysed sample versus the other extraction solvents where the sample became a solid mass. The AOAC buffered salts yielded the cleanest extract, visually and was chosen to be used with the d-SPE containing 50 mg PSA, 150 mg MgSO4 for the extraction of the pharmaceuticals in whole blood. Typical chromatogram shown in Figure 1 is the analysis of a 10 ng/mL whole blood sample after mini-QuEChERS procedure.
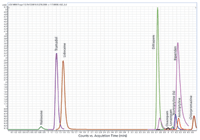
Figure 1: LCâMS-MS chromatograms of 10 ng/mL spiked whole blood sample after mini-QuEChERS extraction; AOAC (NaAc) and d-SPE (PSA).
Method Parameters
Flow Rate: 0.4 mL/min
Mobile Phase: A: 5 mM Ammonium acetate, pH 5; 20:80 MeOH:Water;
B: 5 mM Ammonium Acetate in ACN
Column: Poroshell 120 EC-18, 2.7 μm, 2.1 × 100 mm
Injection: 10 μL
Instrument: Agilent LC 1200, 6460 LC–MS-MS, positive mode,
GT (°C): 300
GF (l/min): 7
Nebulizer (psi): 40
SGT (°C): 400
SFG (l/min): 12
Capillary (V): 3500
NV (V): 500
Gradient: 20 to 75% B over 5.5 min
The mini-QuEChERS approach has shown to be a viable sample preparation technique for the analysis of pharmaceuticals in biologicals like whole blood as shown with average recoveries > 90% and 7% RSDs for 50–100 ng/mL, in Table II.
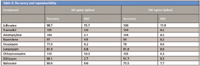
Table II: Recovery and reproducibility
Conclusion
The mini-QuEChERS sample preparation is a simple, easy, and cost effective approach, requiring minimal sample preparation expertise, solvent, equipment, and is a green technology. The mini-QuEChERS approach for the extraction of pharmaceuticals from whole blood offers an alternative sample preparation technique that is easily implemented into laboratories. Poroshell 120 is an excellent column choice for this analysis, in part because it has standard 2 μm frits and is more forgiving for more complex samples relative to a sub-2 μm column. It has mass transfer such that it acts very much like a sub-2 μm particle LC column, but doesn't have the back pressure. The efficient mass transfer equates with faster analysis time and higher throughput, with optimum resolution.
References
(1) M. Anastassiades, S.J. Lehotay, D. Stajnbaher, and F.J. Schenk, J. AOAC Int. 86, 4121 (2003).
(2) G. Stubbings and T. Bigwood, Anal. Chim. Acta 637, 68 (2009).
(3) R.R. Rasmussen, I.M.L.D. Storm, P.ZH. Rasmussen, J. Smedsgaard, and K.F. Nielsen, Anal. Bioanal. Chem. 397, 765 (2010).
(4) D. Smith and K. Lynam, GC/μECD analysis and confirmation of PCBs in fish tissue with Agilent J&W DB-35ms and DB-XLB GC columns Agilent Technologies, 5990-6236EN, (2010).
(5) F. Plössl, M. Giera, and F. Bracher, J. Chrom. A 1135, 19 (2006).
Agilent Technologies, Inc.
2850 Centerville Road, Wilmington, DE 19808
tel. (800) 227-9770, fax (302) 633-8901
Website: www.agilent.com

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.




















