Separation of Quaternary Ammonium Compounds Using a Bonded Polymeric Zwitterionic Stationary Phase
The Application Notebook
Using a bonded polymeric zwitterionic stationary phase, in HILIC mode, polar quaternary herbicides like Diquat can easily be retained, with good peak symmetry. The impact of ionic strength and organic content in the mobile phase is discussed.
Using a bonded polymeric zwitterionic stationary phase, in HILIC mode, polar quaternary herbicides like Diquat can easily be retained, with good peak symmetry. The impact of ionic strength and organic content in the mobile phase is discussed.
Diquat (6,7-Dihydrodipyrido[1,2-a:2',1'-c]pyrazinediium dibromide) is an extremely polar compound, Figure 1, and commonly used as a nonselective contact herbicide. Diquat is a doubly charged cationic analyte. Different separation techniques have been used over time like gas chromatography, electrophoresis, and reversed phase (RP) liquid chromatography. The most common procedure used to be RP-chromatography with addition of ion-pairing (IP) reagents. Most RP-columns are silica based, where presence of residual silanol groups imposes secondary interactions, causing peak tailing. IP reagents are added to the mobile phase to enhance the retention, to mask unwanted secondary interactions, and to improve the analyte peak shape. Presence of IP reagents in the mobile phase decreases the sensitivity, especially when combined with mass spectrometric (MS) detection. IP reagents often show an effect of ion-suppression, decreasing the quantity of ions that reaches the MS. To aid better detection, alternative separation techniques are therefore sought.
An attractive alternative is hydrophilic interaction liquid chromatography (HILIC), where a polar stationary phase is used in combination with aqueous–organic mobile phases. In HILIC mode, the elution order is usually the opposite of reversed-phase chromatography, offering an increased retention of polar and hydrophilic solutes. This means that IP reagents are not needed and coupling to MS detection is eased. This application note focuses on the retention of diquat on a polymeric bonded zwitterionic HILIC stationary phase, the effect of ionic strength and percentage of the organic content in the mobile phase is discussed.
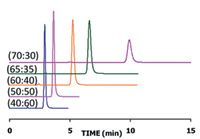
Figure 1: Separation of diquat on a ZIC-pHILIC column; 150 à 4.6 mm, 5 µm. Mobile phase consisted of acetonitrile/250 mM of ammonium acetate pH 3.7 (X:Y, v/v).
Experimental Conditions
Column: ZIC®-pHILIC 150 × 4.6 mm, 5 µm
Mobile phase compositions are given in each figure legend.
Flow rate: 1.0 mL/min
Temperature: 40 °C
Detection: UV at 313 nm
Sample: Diquat, 50 ppm diluted in acetonitrile/water (1:1; v/v)
Injection volume: 5 µL
Results
A polymer-based HILIC column, with a bonded zwitterionic stationary phase was chosen for the separation of diquat, to alleviate silanol interactions with the model compound. After initial scouting experiments, a mobile phase with acetonitrile and ammonium acetate (40:60; v/v) was selected. The pH was set at 3.7, and the impact of ionic strength was evaluated. Due to the electrostatic interactions between diquat and the stationary phase, the retention was strong at lower ionic strength, and as the salt concentration was raised, narrower, more symmetrical peaks were attained along with overall shorter retention. To further optimize the separation, the proportion of organic solvent was varied (see Figure 1). The retention factor for diquat increased from 1.5 to 7 when the acetonitrile content was raised from 40 to 70 volume percent in the mobile phase. The peak symmetry was very good for all compositions; hence it was easy to modulate the retention and to obtain useful experimental conditions for any detection mode.
Conclusion
Separation of extremely polar compounds like diquat is straightforward using a bonded polymeric zwitterionic stationary phase. Altering ionic strength and organic content in the mobile phase, the retention of diquat can easily be modulated and to allow for sensitive detection.
Reference
(1) Dr. Norikazu Nagae, ChromaNik Technologies Inc., Japan.
EMD Chemicals, Inc., An affiliate of Merck KGaA
480 South Democrat Road, Gibbstown, NJ 08027
tel. (800) 222-0342; Website: www.emdchemicals.com

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.





















