Characterization of an Unknown Cannabinomimetic Compound in an Herbal "Incense" Sample by Gas Chromatography–High Resolution Time-of-Flight Mass Spectrometry
The Application Notebook
Synthetic cannabis products have garnered a great deal of media attention recently. Countless products are being sold at smoke shops, convenience stores, and online sites.
Synthetic cannabis products have garnered a great deal of media attention recently. Countless products are being sold at smoke shops, convenience stores, and online sites. These products are labeled "not for human consumption," but have been reported to have effects similar to cannabis when smoked. These reports have prompted testing in crime laboratories across the country. Some of the products tested have been confirmed to contain synthetic cannabinoids. The identification of these compounds by GC–MS can be challenging, as most of them are not present in commercially available mass spectral libraries. A particular product that received a great deal of media attention in the midwestern United States during 2010 was "Mr. Smiley." This product was reported to contain natural substances such as Mullein and Damiana leaf. It was also reported that when smoked, this product exhibited a "THC-like" high. Shortly before this product was pulled from store shelves, some was purchased for analysis to determine which compounds may be present.
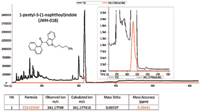
Figure 1: GCâHRT TIC and extracted ion chromatogram for the analyte identified as 1-pentyl-3-(1-naphthoyl)indole or JWH-018. Notice a mass accuracy of 0.2 ppm was achieved.
The sample was initially analyzed by GC–TOFMS, but the most abundant analyte detected was not present in the commercially available NIST mass spectral database.
This application note shows the use of LECO's Pegasus GC–HRT high resolution time-of-flight mass spectrometer to provide an elemental composition based on accurate mass data, which ultimately lead to identification of the unknown compound.
Sample
A 1-gram sample of "Mr. Smiley" was weighed into a 20-mL vial. A 10-mL aliquot of ethyl acetate was added and the sample was sonicated for 10 min.
Experimental
The "Mr. Smiley" extract was analyzed on a LECO Pegasus GC–HRT system using the conditions shown below.
GC: Agilent 7890
Injection: 1 μL, split 50:1 at 275 °C
Column: Rtx-5, 10 m x 0.18 mm x 0.2 μm
Carrier gas: helium at 1.0 mL/min
Oven: 60 °C to 330 °C @ 50 °C/min, hold 10 min
Transfer Line: 300 °C
MS: LECO Pegasus GC–HRT
Acquisition Delay: 120 s
Saved mass range: 40–550 m/z
Flight Path: High Resolution (R = 25,000 FWHM)
Acquisition rate: 20 spectra/s
Source temperature: 250 °C
Results
The accurate mass data provided by the Pegasus GC-HRT was consistent with the formula C24H23NO. A literature search lead to identification of this formula as 1-pentyl-3-(1-naphthoyl)indole, or JWH-018, one of the most common synthetic cannabinoids used in "herbal incense" products.
Conclusions
This application shows how the high resolving power and mass accuracy of the Pegasus GC–HRT facilitate identification of substances absent from commercially available mass spectral libraries. This ability will be required as new abused substances are continually developed.
LECO Corporation
3000 Lakeview Avenue, St. Joseph, MI 49085
tel. (269) 985-5496, fax (269) 982-8977
Website: www.leco.com

A Matrix-Matched Semiquantification Method for PFAS in AFFF-Contaminated Soil
Published: April 14th 2025 | Updated: April 14th 2025Catharina Capitain and Melanie Schüßler from the Faculty of Geosciences at the University of Tübingen, Tübingen, Germany describe a novel approach using matrix-matched semiquantification to investigate per- and polyfluoroalkyl substances (PFAS) in contaminated soil.
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.




















