Hyaluronic Acid (Polysaccaccharides)
The Application Notebook
Wyatt Technology corporation
Hyaluronic acid (HA) is an ubiquitous, very high molar mass polysaccharide that has been of particular importance in opthalmic surgery. HA acts as a molecular "shock-absorber" and stabilizer for cells and its visco-elastic properties are valuable for separating tissue and maintaining shape. It is a critical component in tissue lubrication and is believed to play a leading role in wound repair. Finally, HA's property of non-pyrogenicity makes it an ideal sheath for implants, whose presence might cause the body to suffer an immune response.
Among the many benefits high molecular weight HA hold are
- the maintenance of tissue space for surgery
- the protection of cells and tissue
- therapeutic effectiveness.
HA's therapeutic effectiveness depends critically on molecular weight: the higher the molecular weight, the longer its benefit. However, because of its visco-elastic properties, standards-based GPC analysis is inappropriate for characterizing HA. There exist no standards identical to HA and the desirability of altering experimental conditions renders conventional GPC/SEC impractical.
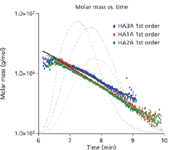
Figure 1: From the molar mass versus time plot, subtle differences can be seen among the samples.
Combining a DAWN with HPLC separation, however, provides an ideal platform for absolute characterization, since the light-scattering measurements do not depend on pump speed, polymer standards or molecular conformation.
A DAWN was connected to a GPC/SEC line (100 mM NaN03 buffer, TSK-Gel G6000PW column, Optilab DSP refractometer, Waters 510 pump) and generated the data required to determine not only absolute molar mass, but also molecular size, for a variety of HA products.
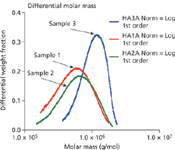
Figure 2: The differential molar mass distributions calculated by ASTRA immediately confirm the large differences among the three HA samples.
Figure 1 shows the molar mass versus time (with the 90° lightscattering chromatograms in the background) for the three samples, ranging from approximately 2 million to less than 200K Daltons. Figure 2 illustrates how profoundly different the samples are by revealing their differential molar mass distributions. These indicate that the samples will behave in different ways when used medically, depending on the content of their high molecular weight HA.
Wyatt Technology Corporation
6300 Hollister Avenue, Santa Barbara, California 93117, USA
tel. +1 (805) 681 9009 fax +1 (805) 681 0123
E-mail: info@wyatt.com Website: www.wyatt.com

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.




















