High Speed, High Resolution Analysis of Aflotoxins
The Application Notebook
Shimadzu
Aflatoxins are toxins produced by several aspergillus fungus species. As well as being highly toxic, they are also known to be carcinogenic, so the inspection of foods for their presence is necessary (1). In Europe the maximum allowed concentrations in food and food compositions are regulated; limits have been described for the totals and for the species aflatoxin B1, B2, G1 and G2. This application describes two cases of analysis of these 4 aflatoxins (B1, B2, G1 and G2), using the prominence RF-20AXS high-sensitivity fluorescence detector while applying trifluoroacetic acid derivatization and direct detection.
Derivatization of Aflatoxins with Trifluoroacetic Acid
Typically, aflatoxins B1 and G1 are converted to the hydroxylderivative aflatoxins B2a and G2a using trifluoroacetic acid (TFA) to increase their fluorescence intensity in HPLC analysis. Figure 1 shows the structures of the 4 aflatoxins and the B2a and G2a TFA derivatives. When analysing aflatoxins in food, TFA derivatization is done for both the standard solution and the sample solution.
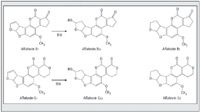
Figure 1: Structures of aflatoxins B1, B2, G1, G2 and trifluoroacetic acid-derivatized forms (B2a, G2a).
Analysis of Standard Solution after Derivatization with Trifluoroacetic Acid
Figure 2 shows chromatograms of standard solutions of the 4 aflatoxins (B1, B2, G1 and G2) obtained following TFA derivatization (Figure 5) with 20 µL injections, and Table 1 shows the analytical conditions. A peak area repeatability (n = 6) of 1.2% relative standard deviation (RSD) was obtained for the B2a peak in the chromatogram on the right in Figure 2, and the detection limit (S/N ratio = 3.3, 20 µL injection) was calculated to be 0.4 ng/L (8 fg). Figure 3 shows the calibration curves (B1 and G1: 0.004–20 µg/L, B2 and G2: 0.001–5 µg/L). Excellent linearity was obtained for all 4 components, with an R2 value greater than 0.9999. Ultra-trace levels of aflatoxins can therefore be detected accurately with high sensitivity using the RF-20AXS.

Figure 2: Chromatograms of aflatoxin standard solutions after derivatization with trifluoroacetic acid (20 µL injected); (left) B1 and G1: 2.0 µg/L, B2 and G2: 0.5 µg/L, (right) B1 and G1: 20 ng/L, B2 and G2: 5 ng/L.
Table 1: Analytical conditions.
Column: Shim-pack FC-ODS (150 mm L. × 4.6 mm i.d., 3 µm)
Mobile phase: Water/Methanol/Acetonitrile = 6/3/1 (v/v/v)
Flow rate: 0.8 mL/min
Column temp.: 40 °C
Detection : RF-20AXS, Ex at 365 nm, Em at 450 nm
RF cell : Conventional cell
Cell temp.: 25 °C
Injection volume: 20 µL

Figure 3: Calibration curves of aflatoxin standard solutions after derivatization with trifluoroacetic acid (B1 and G1: 0.004â2.0 µg/L, B2 and G2: 0.001â5 µg/L, 20 µL injected).
Apart from using derivatization methods, direct detection of aflatoxins is also possible using the highly sensitive RF-20AXS fluorescence detector.
Analysis of Standard Solution by Direct Detection
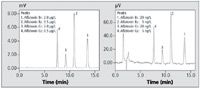
Figure 4: Chromatograms of aflatoxin standard solutions by direct detection (20 µL injected) (left) B1 and G1: 2.0 µg/L, B2 and G2: 0.5 µg/L, (right) B1 and G1: 20 ng/L, B2 and G2: 5 ng/L.
Figure 4 shows the chromatograms of standard solutions of the 4 aflatoxins (B1, B2, G1, G2) obtained with 20 µL injections without TFA derivatization. Analytical conditions were the same as those shown in Table 1. Regarding the B1 peak in the chromatogram on the right in Figure 4, a peak area repeatability (n = 6) of 2.7% RSD was obtained, and the detection limit (S/N ratio = 3.3, 20 µL injection) was calculated to be 3 ng/L(60 fg). This demonstrated that testing for aflatoxin B1 and G1 can be done with sufficient sensitivity using direct detection with the RF-20AXS, without TFA derivatization.
Analysis of Food
For a food application example, aflatoxin standard solution was added to commercially available cornflour so that the aflatoxin concentrations in the sample became 0.8 µg/kg for B1 and G1, and 0.2 µg/kg for B2 and G2, respectively. The pseudo-contaminated sample was then analysed using both TFA derivatization and direct detection without derivatization. Figure 5 shows the chromatograms obtained from analysis of commercially available cornflour, unspiked (blank) and spiked with the aflatoxin standard solution, in this case using TFA derivatization.
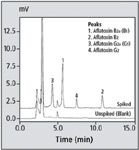
Figure 5: Chromatograms of cornflour using derivatization with TFA (20 µL injected) (upper) spiked with aflatoxin standard, (lower) unspiked.
Reference
(1) Shimadzu - LC Application News L 428, Shimadzu Corp. Kyoto, Japan.
Shimadzu Europa GmbH
Albert-Hahn-Strasse 6-10 47269 Duisburg, Germany
tel. +49 203 7687 0 fax +49 203 766625
E-mail: shimadzu@shimadzu.eu
Website: www.shimadzu.eu

Altering Capillary Gas Chromatography Systems Using Silicon Pneumatic Microvalves
May 5th 2025Many multi-column gas chromatography systems use two-position multi-port switching valves, which can suffer from delays in valve switching. Shimadzu researchers aimed to create a new sampling and switching module for these systems.
Studying Cyclodextrins with UHPLC-MS/MS
May 5th 2025Saba Aslani from the University of Texas at Arlington spoke to LCGC International about a collaborative project with Northwestern University, the University of Hong Kong, and BioTools, Inc., investigating mirror-image cyclodextrins using ultra-high performance liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS) and vibrational circular dichroism (VCD).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)


















