Analysis of "Broad Spectrum" UVA and UVB Components in Sun Care Products for Compliance with New FDA Regulations
Perkin Elmer
The FDA has made changes to how products containing sunscreen are labeled in the U.S. to ensure they meet the new regulations set forth for safety and effectiveness. The new regulations will require companies that want to use the "broad spectrum" label to test for both UVA and UVB protection. Previous regulations only dealt with preventing sunburn which is primarily due to UVB radiation but did not address UVA which protects against early ageing and skin cancer. All products that claim to provide broad spectrum SPF protection are regulated as sunscreen products. Therefore, the regulations the FDA has developed for over the counter (OTC) sunscreen products apply to cosmetics, moisturizers, lip balms and shampoos labeled with SPF values.

Table 1: Experimental Conditions.
Results
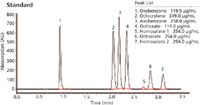
Figure 1: All UVA and UVB compounds are eluted in a single run using a Brownlee SPP column with an optimized PerkinElmer HPLC system.
Conclusion
The methodology in Figure 1 provides one test method to determine both UVA and UVB compounds most commonly used in the sun care industry. Figure 2 is a sun care spray run with the same analytical conditions eluting six UVA/B compounds. All UVB and UVA compounds are eluted in a single run and can be quantitated in the ranges recommended by the FDA (SPF 15–50+). Only broad spectrum products with an SPF value of 15 or higher can claim to reduce the risk of skin cancer and early skin ageing if used as directed with other sun protection measures.
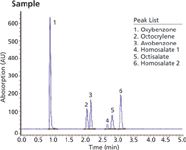
Figure 2: SPF 50 sun care spray shows elution of both UVA/B compounds.
References
(1) U.S. Food and Drug Administration, 2012. Information for Consumers, 2012. Retrieved from http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/ucm258468.htm.
PerkinElmer
940 Winter Street, Waltham, Massachusetts 02451, USA
tel. (800) 762 4000 fax (203) 944 4904
Website: www.perkinelmer.com





















