Challenges in Developing an Ultra Sensitive Bioanalytical Method for Ethinylestradiol in Human Plasma
The Application Notebook
Waters Corporation
Introduction
Ultra sensitive LC–MS-MS quantification of steroids is particularly difficult due to the presence of countless other steroid interferences, many isobaric, in human plasma. In particular, for compounds such as ethinylestradiol (EE), where the required detection limit (LOD) is 1 pg/mL, this challenge is even greater. Not only must one use the highest sensitivity MS system, but both LC and sample preparation must also be carefully optimized for selectivity.
Instrumentation & Consumables
LC Conditions
Instrument: ACQUITY UPLC®
Column: ACQUITY UPLC HSS C18 SB, 2.1 × 100 mm, 1.8 μm
Part number: 186004119
Mobile phase A: 0.1% HCOOH in H2O
Mobile phase B: 0.1% HCOOH in 80/20 ACN/IPA (v/v)
Initial mobile phase conditions were 60% mobile phase B (MPB). After a 1 min hold, the percentage of MPB was increased to 90% over 4 min. The flow rate was 0.4 mL/min. The injection volume was 35 μL.
Sample Preparation Conditions
Human plasma samples were extracted with 75/25 hexane/ethyl acetate (v/v), derivatized with dansyl chloride, and then the derivatives cleaned up with Oasis® MCX in μElution format (part number 186001830BA.)
MS Conditions
Instrument: Xevo® TQ-S
Ion Source: ESI+
Results/Discussion
Figure 1 demonstrates the importance of a multi-step clean-up process for high sensitivity analysis of EE. Following liquid-liquid-extraction (LLE) alone, the MS scan in red clearly highlights the overwhelming level of interferences remaining. This interference peak not only co-elutes with EE, but also saturates the detector. These interferences prohibit one from reaching the desired LOD. The interferences present after LLE alone contain a high concentration of phospholipids amongst other things. When LLE is followed by mixed-mode cation exchange (Oasis MCX –green trace), the interferences are practically eliminated, allowing one to reach the required detection limits.

Figure 1: Representative MS scan data for human plasma extracted using liquid-liquid-extraction (LLE) alone (red) and LLE followed by mixed-mode SPE (green). The retention time for EE is shown in purple.
The chromatograms in Figure 2 demonstrate that one can easily meet the detection limit of 1 pg/mL in human plasma, as the level in panel A is > 3× the level in the blank extracted plasma sample (panel B). To assess accuracy and precision, standard curves were prepared from 0.001–1 ng/mL. Quality control (QC) samples were prepared at low, medium and high concentrations: 0.003, 0.075 and 0.75 ng/mL, respectively. Regression analysis of the data produced standard curves with an r2 value of 0.999 using a 1/x weighting. The average % accuracy for the points on the standard curves is 99%. The average % accuracy for the QC samples is 96%.
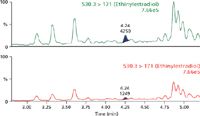
Figure 2: Panel A is a representative chromatogram of EE at 1 pg/mL in extracted human plasma compared to Panel B, which is the blank extracted plasma.
Conclusions
The proper combination of optimized sample prep and chromatography coupled to high sensitivity MS produces a bioanalytical method for EE which reaches an LOD of 1 pg/mL in human plasma.
For full application note, visit www.waters.com/4295
©2012 Waters Corporation. Waters, The Science of What's Possible, ACQUITY UPLC, Oasis and Xevo are trademarks of Waters Corporation.
Waters Corporation
34 Maple Street Milford, Massachusetts 01757, USA
Tel. +1 508 478 2000 fax +1 508 872 1990
Website: http://www.waters.com

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.





















