Rapid On-Line-SPE HPLC Determination of Carbofuran and Carbaryl in Tap and Environmental Waters
The Application Notebook
Thermo Scientific
N-Methylcarbamates are widely used agricultural pesticides. For their determination, reversed-phase high-performance liquid chromatography (RP-HPLC) with fluorescence detection following postcolumn derivatization, per U.S. EPA Methods 531.2 and 8318, is typically used. When using HPLC with UV detection, a sample preparation procedure — either liquid–liquid extraction or off-line solid-phase extraction (SPE)—is required to increase detection sensitivity. However, these procedures are time-consuming, require large volumes of organic solvents, and are deficient in terms of process control. This work describes an automated online SPE HPLC with UV absorbance method that provides a rapid determination of carbofuran and carbaryl, two of the most frequently used carbamate pesticides, without the need for postcolumn derivatization.
Method Conditions and Sample Preparation
The experimental configuration, sample preparation procedures, more experimental results and references are described in Thermo Scientific Application Update 186.
Results
Linearity was tested using standards with concentrations of 0.5–100 µg/L undergoing on-line SPE under the specified chromatographic conditions (r value better 0.9999). The method showed excellent sensitivity, with detection limits better than those defined in the EPA Method 531.2 (carbofuran 0.062 µg/L, carbaryl 0.036 µg/L), in the standard method enacted by the Ministry of Health, People's Republic of China (7 µg/L for carbofuran), as well as other applicable U.S. and European drinking water regulations. Reproducibility for a 5 µg/L spiked standard was very good, with ca. ±1% area RSD.
Trace B in Figure 1 illustrates good separation and response for carbofuran and carabryl using a 2500 µL injection volume. Here, tap water was spiked with 1 µg/L carbofuran and carbaryl. Injection volumes larger 2500 µL were not beneficial, as an overloading of the SPE cartridge was observed (Figure 1 Trace A). Spike experiments using tap, pond, surface, and farmland water from the Pudong District of Shanghai, China showed excellent SPE recoveries between 81–120% for a 2500 µL injection volume. Resolution between carbofuran and carbaryl was 3.5, exceeding the value required by the EPA Methods ≤1.0). A Thermo Scientific Dionex SolEx on-line SPE HRP cartridge, 12–14 µm, 2.1 × 20 mm, was used for the enrichment. A Thermo Scientific Acclaim 120 C18, 3 µm Analytical, 3 × 150 mm column was used for the separation. Under the optimized chromatographic conditions, the complete analysis required only 5 min.
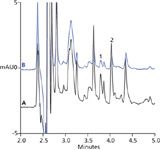
Figure 1: Chromatograms of a tap water sample spiked with 1 µg/L each carbofuran and carbaryl standards: A) 10,000 µL; and B) 2500 µL injection volumes.
Conclusion
Fully automated on-line SPE HPLC as optimized and illustrated here provided good selectivity and suitability for the rapid analysis of carbofuran and carbaryl in tap and environmental water samples. With excellent linearity, sensitivity, and reproducibility, on-line SPE HPLC provides full automation, eliminates operator-related variation and can help enforce strict process control.
Receive the complete application note at: www.thermoscientific/AU186
Thermo Fisher Scientific Inc.
1228 Titan Way, P.O. Box 3603, Sunnyvale, California, USA
tel. (800) 532 4752
Website: www.thermoscientific.com/dionex

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
Investigating the Protective Effects of Frankincense Oil on Wound Healing with GC–MS
April 2nd 2025Frankincense essential oil is known for its anti-inflammatory, antioxidant, and therapeutic properties. A recent study investigated the protective effects of the oil in an excision wound model in rats, focusing on oxidative stress reduction, inflammatory cytokine modulation, and caspase-3 regulation; chemical composition of the oil was analyzed using gas chromatography–mass spectrometry (GC–MS).



















