Improved Resolution of Triazine Herbicides in Drinking Water and Wastewater
The Application Notebook
Phenomenex Application Note
Often used for weed control, triazine herbicides have been found to have detrimental environmental and health effects. Much debate has focused on the level at which these compounds negatively impact health. To monitor and control human exposure to these herbicides, regulatory bodies have established allowable limits of triazines in drinking and wastewater.
In this study, two methods for triazine herbicide analysis are presented. They follow the Environmental Protection Agency's Method 536 for drinking water and Method 619 for wastewater, using LC–MSMS and GC–MS respectively.

Table 1: LCâMS-MS operating conditions.
Experimental Conditions
For EPA Method 536, an Applied Biosystems API 3000 LC/MS/MS was used. Operating parameters are displayed in Table 1. For EPA Method 619, an Agilent 6890/5975 was used. Operating parameters are displayed in Table 2.
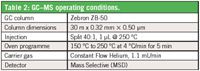
Table 2: GCâMS operating conditions.
Results
LC–MS-MS provides the sensitivity needed to accurately quantitate and verify the identity of triazine herbicides in EPA Method 536. The conditions presented provide separation of the herbicides in less than 6 min, as shown in Figure 1. The narrow peaks observed result from the ultra-high efficiency of the Kinetex® 2.6 µm XB-C18 column. The short run time improves laboratory productivity and minimizes sample backlogs.
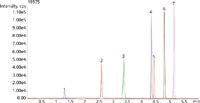
Figure 1: Resultant LCâMS-MS chromatogram of EPA Method 536 using Kinetex 2.6 µm XB-C18.
EPA Method 619 uses gas chromatographic means to detect a longer list of triazine herbicides by GC–MS, as shown in Figure 2. The Zebron™ ZB-50 column provides separation of all compounds, delivering confidence in qualitative and quantitative results.
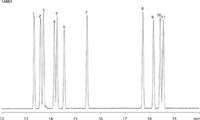
Figure 2: Resultant chromatogram of EPA Method 619 using Zebron ZB-50.
Conclusions
Two successful methods have been presented to monitor triazine herbicides. The narrow peaks achieved with the Kinetex 2.6 µm XB-C18 core-shell technology column increased throughput without sacrificing resolution for LC-based EPA Method 536. The Zebron ZB-50 column provides separation of all 11 triazine herbicides for GC-based EPA Method 619.
Phenomenex Inc.
411 Madrid Avenue, Torrance, California 90501, USA
tel. +1 (310) 212 0555 +1 (310) 328 7768
Website: www.phenomenex.com

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.




















