Fast Separation of Seven Biocides Using an Agilent ZORBAX RRHD Eclipse Plus C18 1.8 µm Column
The Application Notebook
The flexibility to use short sub-2 micron columns on UHPLC instruments at high flow-rates permits fast analysis with high resolution of complex samples. An Agilent ZORBAX Rapid Resolution High Definition (RRHD) Eclipse Plus C18 1.8 µm column separated seven different biocides in less than 1 minute with high resolution. A flow-rate of 1.7 mL/min was used on 2.1 × 50 mm column to achieve the rapid separation. The Agilent 1290 Infinity LC system was used as the column pressure just exceeded 1000 bar at this high flow-rate. A sample of hand sanitizer was found to contain 2-phenoxyethanol and methylparaben using the method.
The flexibility to use short sub-2 micron columns on UHPLC instruments at high flow-rates permits fast analysis with high resolution of complex samples. An Agilent ZORBAX Rapid Resolution High Definition (RRHD) Eclipse Plus C18 1.8 µm column separated seven different biocides in less than 1 minute with high resolution. A flow-rate of 1.7 mL/min was used on 2.1 × 50 mm column to achieve the rapid separation. The Agilent 1290 Infinity LC system was used as the column pressure just exceeded 1000 bar at this high flow-rate. A sample of hand sanitizer was found to contain 2-phenoxyethanol and methylparaben using the method.
Introduction
Biocides are used to control harmful organisms such as bacteria, fungi and rodents and help protect health, improve product performance and prevent spoilage. They are found in many areas including medicine, agriculture, forestry and in industry and are highly regulated because of their potential health risk to humans or the environment. The demand for biocides will continue to increase as the public and consumers' awareness of the benefits of continued improvements in hygiene grows. Many biocides are synthetic and this work includes the fast separation of seven different synthetic biocides used in various applications.
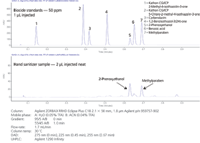
Figure 1: Rapid UHPLC separation of biocides and analysis of hand sanitizer on Agilent ZORBAX RRHD columns.
Experimental
Instrumentation and chromatographic parameters are found in Figure 1. A 100 ppm stock solution (50/50 ACN/H2O) of the biocide standards was diluted with 5% ACN in H2O to make a 50 ppm standard mix.
Results and Discussion
Seven different biocides were easily separated in only 0.7 minutes on an RRHD Eclipse Plus C18 1.8 μm column as shown in Figure 1. All compounds were baseline resolved with a minimum resolution factor of 1.8. A very fast linear gradient and high flow-rate of 1.7 mL/min on the 50 mm RRHD column produced pressure over 1000 bar. RRHD columns are designed for UHPLC systems such as the Agilent 1290 Infinity operating at pressures up to 1200 bar. UV detection included programmed wavelength switching to maximize detection response and improve sensitivity. Analysis of all standards showed linearity from 3 to 25 ppm.
Hand sanitizer was analysed as shown in the bottom half of Figure 1. The sanitizer was injected neat (2 μL) with no detrimental effects. Two biocides, 2-phenoxyethanol and methylparaben, which are common preservatives in personal care products, were identified without difficulty with the fast method. To assist in MS detection, 0.1% formic acid can be substituted for the trifluoroacetic acid.
Conclusion
The ZORBAX RRHD Eclipse Plus C18 1.8 μm column was used in conjunction with the Agilent 1290 Infinity LC for a fast, high resolution separation of biocides. The rapid analysis method was successfully applied to the separation and identification of two biocides in a hand sanitizer. Benefits include speed with high resolution for improved productivity.
References
Derek J. Knight and Mel Cooke, "The Biocide Business: Regulation, Safety and Applications," Wiley-VCH 2002.

Agilent Technologies Inc.
2850 Centerville Road, Wilmington, Delaware 19808, USA
tel. +1 800 227 9770 fax +1 302 633 8901
E-mail: info_agilent@agilent.com Website: www.agilent.com/chem

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
Investigating the Protective Effects of Frankincense Oil on Wound Healing with GC–MS
April 2nd 2025Frankincense essential oil is known for its anti-inflammatory, antioxidant, and therapeutic properties. A recent study investigated the protective effects of the oil in an excision wound model in rats, focusing on oxidative stress reduction, inflammatory cytokine modulation, and caspase-3 regulation; chemical composition of the oil was analyzed using gas chromatography–mass spectrometry (GC–MS).


















