Simplifying Peptide Bioanalysis
The Application Notebook
Streamlined sample prep, LC and MS method development.
Erin E. Chambers, Jessalynn Wheaton and Diane M. Diehl, Waters Corporation, Milford, Massachusetts, USA.
Highlights
- Streamlined sample prep, LC and MS method development.
- Complete LC and SPE screening protocols provided.
- 15× sample concentration without evaporation.
Analytical Challenges
- Losses due to poor solubility and adsorption make sample concentration without evaporation highly desirable.
- Obtaining selectivity for peptide therapeutics in complex biological matrices is crucial in order to minimize matrix effects and reduce variability associated with incurred samples.
- Sensitivity by mass spectrometry is often lower for biomolecules than typical small molecules, necessitating sample concentration and use of the highest sensitivity LC and MS methodologies.
- An MS system with an adequate mass range is required for detection of higher m/z precursors and fragments.
Key Aspects of the Solution
A Peptide Separation Technology (PST) method development kit was was developed and is available for ACQUITY UPLC (part#176001835) or HPLC (part#176001836.) The kit contains a chromatographic column, the Oasis μElution PST method development extraction plate and simple, logical SPE and LC screening protocols. ACQUITY UPLC technology was used to increase sensitivity, resolution and separation speed. Representative LC–MS performance using the UPLC screening protocol from the kit is shown in Figure 1. It should be noted that a critical pair, vasopressin and desmopressin (differing only by an amino group), are well separated using the generic ACQUITY UPLC method.

Figure 1
Samples were extracted using the Oasis μElution PST method development extraction plate according to the generic screening protocol included in the kit. SPE results are shown in Figure 2. The Xevo TQ MS was chosen for this application because the mass range on both quadrupoles is m/z 2048, a key requirement for bioanalysis of a wide range of peptides. Figure 3 demonstrates a representative LLOQ for angiotensin I extracted from human plasma using the generic LC and SPE screening protocols without optimization.

Figure 2
Conclusion
The Oasis PST μElution method development plate and protocol can greatly simplify extraction method development and rapidly identifies an appropriate SPE sorbent and selective starting protocol for peptide bioanalysis. The combination of ACQUITY UPLC coupled with the mass range, speed and sensitivity of Xevo TQ MS provides the LC–MS performance required for peptide bioanalysis.
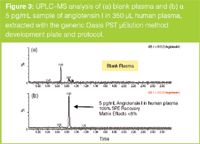
Figure 3
©2010 Waters Corporation. Waters, The Science of What's Possible, ACQUITY UPLC, UPLC, Oasis, Xevo are trademarks of Waters Corporation.
For the complete UPLC application note, visit www.waters.com/3253

Waters Corporation
34 Maple Street Milford, Massachusetts 01757, USA
tel. +1 508 478 2000 fax +1 508 872 1990

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.














