BTEX Determination in Gasoline Containing Ethanol by Single Oven MDGC
The Application Notebook
The determination of BTEX in gasoline is usually performed in accordance with the standard test method ASTM D3606 using gas chromatography. However, particularly in the presence of ethanol, co-elutions are observed in one-dimensional chromatography. In this application note a method is described applying multidimensional GC to overcome the separation problem.
Introduction
The determination of BTEX in gasoline is usually performed in accordance with the standard test method ASTM D3606 using gas chromatography. However, particularly in the presence of ethanol, co-elutions are observed in one-dimensional chromatography. In this application note a method is described applying multidimensional GC to overcome the separation problem.
Multidimensional GC
In multidimensional GC two columns of different properties are coupled to each other. In this way, compounds that co-elute on one stationary phase can be separated on another column, as only this part of the chromatogram is sent to the second column and detector. Separation of other compounds in the sample remains unchanged and they are monitored at the first detector.
By using the recently developed Multi-Deans Switching technique the outlet pressure for the first column remains unchanged even when switching is performed. This enables an easy multiple heart-cut method set-up without any retention time shifts in the monitor chromatogram.
Instrumentation
GC-2010 with MDGC switching package and two FID-2010
Experimental Conditions
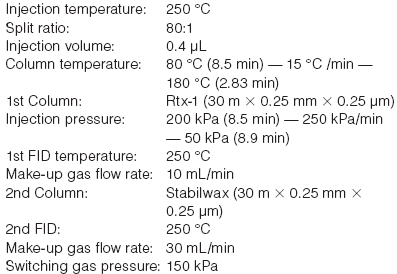
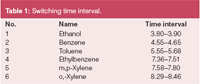
Table 1: Switching time interval.
Results
Figure 1 shows a chromatogram of a gasoline sample containing ethanol and BTEX monitored on the first FID (no cuts).
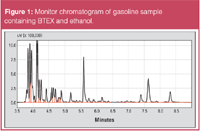
Figure 1
If the same sample is measured in cut mode, the following chromatogram is obtained on the 2nd FID (Figure 2).
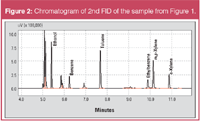
Figure 2
To obtain a good chromatogram on the 2nd FID it is important that retention time shifts do not occur on the 1st chromatogram, otherwise definition of the correct cut times is very difficult.
By using the recently developed Multi-Deans Switching technique the outlet pressure for the first column remains unchanged even when switching is performed. This enables an easy multiple heart-cut method set-up without any retention time shifts in the monitor chromatogram.
Figure 3 shows a comparison of the monitor chromatogram on the 1st FID with (purple) and without (black) cutting to the 2nd FID. It is clear that the retention times for the gasoline peaks are the same, regardless of whether cutting occurred or not.
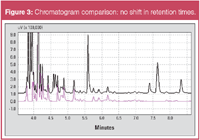
Figure 3
Conclusions
The multidimensional GC method is well suited to the analysis of BTEX and ethanol in gasoline samples. Co-elutions observed in one-dimensional GC can be overcome using a newly developed Multi-Deans Switching device in the column oven, which transfers selected cuts to a second separation column and second FID without any retention time shifts in the first dimension.

Shimadzu Europa GmbH
Albert-Hahn-Str. 6-10, D-47269 Duisburg, Germany
tel. +49 203 76 87 0 fax +49 203 76 66 25
E-mail: shimadzu@shimadzu.eu
Website: www.shimadzu.eu

Fundamentals of Benchtop GC–MS Data Analysis and Terminology
April 5th 2025In this installment, we will review the fundamental terminology and data analysis principles in benchtop GC–MS. We will compare the three modes of analysis—full scan, extracted ion chromatograms, and selected ion monitoring—and see how each is used for quantitative and quantitative analysis.
Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
This information is supplementary to the article “Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing”, which was published in the May 2025 issue of Current Trends in Mass Spectrometry.













