Separation Science: The State of the Art: Nontargeted Screening in Environmental Analysis: An Example Showing Migration of Chemicals from Reusable Sports Plastic Bottles into Drinking Water
Reusable plastic water bottles are popular, especially during sport, due to their lightweight and impact‑resistance properties. However, little is known about the migration of compounds from plastic into the drinking water, as a wide variety of intentionally added substances (IAS) are used in the production of plastic. Furthermore, an unknown number of nonintentionally added substances (NIAS) contribute to the chemical complexity. NIAS are often unknown themselves (1), and can be introduced during the production as impurities, as well as breakdown or transformation products (TPs) caused by multiple stressors such as sunlight, physical use, or elevated temperatures (2).
Bisphenol A (BPA) is one of the chemicals of concern in plastics, and products are nowadays sold as BPA‑free. However, BPA has been replaced by bisphenol S and F, which have been shown to be carcinogenic as well (3). The new prioritization and identification of the regrettable substitutions will take additional time for risk evaluation before they can be phased out. Thus, a more broad screening of known and unknown compounds such as nontargeted screening (NTS), not yet included in regulations or databases, is needed.
In this study, we investigated the chemical migration into drinking water stored for 24 h in new bottles, used bottles, and bottles washed in the dishwasher. NTS by liquid chromatography–high-resolution mass spectrometry (LC–HRMS) was performed to identify these compounds. The experiment was designed to be as close as possible to typical consumer use. The chemical composition of the stored tap water was investigated after each migration step and compounds were prioritized based on systematic changes in peak intensities across the treatments. The entire study is published in the Journal of Hazardous Materials (4).
Nontarget Screening for Prioritization of Chemicals
Using the NTS approach, hundreds of chemicals were analyzed migrating out of the plastic from new and used sports plastic bottles. After a dishwasher cycle of the same bottles, thousands of additional, dishwasher soap-related, compounds migrated into drinking water stored for 24 h. In Figure 1, the 2000 compounds detected in the new bottles are shown in a heat map that shows the origin of the chemicals. Around 100 plastic-related compounds from the new purchased bottles were flushed away after washing the bottles in the dishwasher (a). However, another 50 compounds (b) were still in the drinking water after the dishwasher but were removed after additional flushing. One hundred and seventy-five compounds were observed to migrate out of the bottles consistently (c), even after flushing the bottles with drinking water.
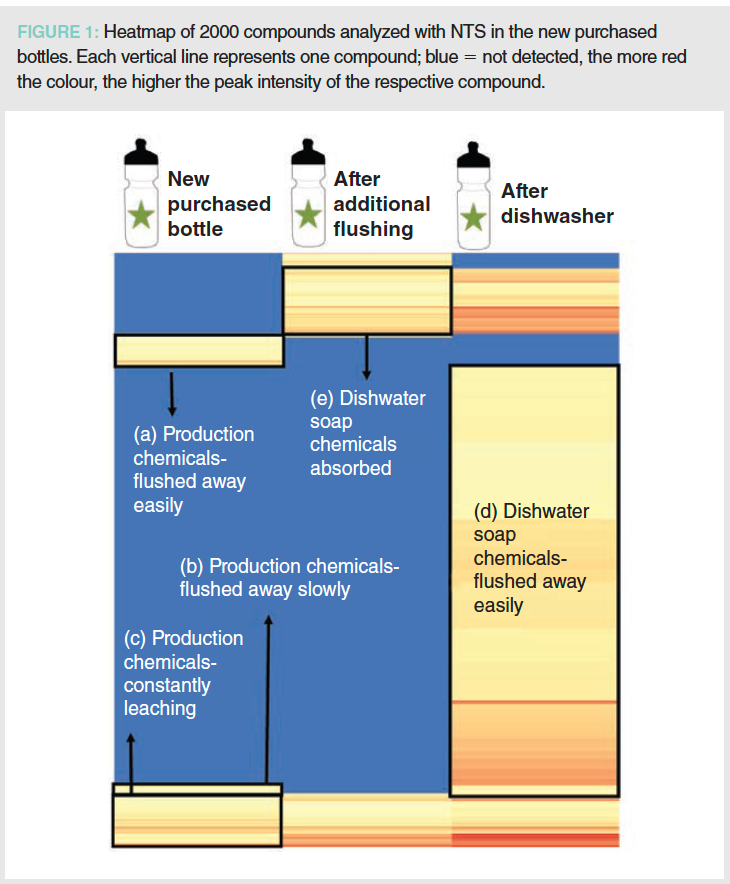
It is of special concern that the concentration of some of these chemicals was even higher in the drinking water after the dishwasher cycle—indicated by vertical lines with more red colour in the row “after the dishwasher”. Most of the detected compounds were derived from the dishwasher soap, and so were detected after the dishwasher cycle (d) but removed after additional flushing of the bottles. Some of the dishwasher soap adsorbed to the material and could not be flushed away by additional water flushing (e).
Dishwasher Soap Adsorbs More to Plastic than to Glass
Surfactants that originated from the dishwasher soap were removed to more than 90% after additional flushing. However, there were several compounds still adsorbed to the plastic surface. The main detergents, which were responsible for the 60 most intense peaks, were identified as homologous series from polyoxyethylen lauryl (C12) ether and polyoxyethylen (C14) ether. These homologue series contained an increasing number of EO groups, with decreasing retention time as a function of polarity. The homologue series showed a removal trend that correlated with the number of EO groups, therefore the higher the number of the EO homologues in the series, the better the removal after additional flushing. The very polar homologue with the highest molecular mass and most EO groups (C12H26O)+(C2H4O)13 was almost completely removed (99% removal of the original intensity left), whereas the most nonpolar homologues (C12H26O)+(C2H4O)4 were on average removed less than 30% after flushing.
DEET—Insect Repellent in Plastic?
The identification of the insect repellent DEET in the plastic bottles was surprising at first glance and shows the strength of NTS. DEET was detected in the water from all plastic bottles, with the highest peak intensity in the older, previously used, bottles. DEET was also detected at low peak intensities in the new bottles but not in the glass bottles nor in the solvent blanks. Therefore, the dishwasher and laboratory blanks could be excluded as a source for DEET. We suspect that DEET, which is a ubiquitous water contaminant (5), has another origin other than its use as an insect repellent. DEET demonstrates the properties of a plasticizer (6), and for that reason it could be introduced in the plastic as a plasticizer itself or it could be formed during the use of the bottles by the degradation of other chemicals. We detected a good correlation between DEET and the plasticizer laurolactam, which has the chemical formula C12H23NO and only 6 H less than DEET. It is possible that some impurities could remain during the laurolactam production process, where there is incomplete desaturation during workup, and therefore a similar compound to DEET could be formed. However, DEET could also have been formed from laurolactam itself during the dishwasher process, with the aid of catalyzing compounds in the plastic (for example, quinones such as anthraquinone).
Other Detected Compounds and Toxicity
We detected >400 plastic-related compounds as well as >3500 dishwasher-related compounds by LC–MS NTS. The chemical structure was identified for around 50 prioritized compounds. Most plastic‑related compounds were plasticizers, antioxidants, and photoinitiators. The highest peaks were detected for oligomers suspected to originate from the biodegradable polyester polycaprolactone. The most concerning compounds were photoinitators because of their high potential adverse effects. Irgacure 369 is an amine co-initiator, a group well-known for their endocrine-disrupting effects, and 4-methylbenzophenone has shown carcinogenicity, reproductive toxicity, and skin contact toxicity in animal testing. Anthraquinone is of concern because of its breakdown products that may be toxic and carcinogenic. The dishwashing process enhanced the migration of plasticizers, antioxidants, and photoinitiators into the drinking water. Therefore, the highest predicted toxic hazard was calculated (by Cramer’s rule) for the used plastic bottles that had been filled with drinking water after the dishwasher and without further flushing. The toxicity decreased after additional flushing.
Due to the lack of analytical standards in NTS, no concentration of the identified compounds could be determined. Therefore, the risk of the stored drinking water in these reusable bottles currently remains unknown. More research is needed to identify and quantify more of the unknown unknowns detected by NTS, as well as toxicological tests to assess the relevance and risk of the newly identified compounds. The study raises the question as to whether plastic bottles are suitable for re-use, especially when they are labelled as biodegradable plastic. The study emphasizes that the production of biodegradable plastic bottles does not mean that the bottles are necessarily made of naturally occurring compounds. Instead, it can be speculated that plasticizers will migrate more easily into consumer drinking water when the biodegradable plastic bottles slowly degrade during use.
Other Uses of NTS in Environmental Analysis
NTS have been and are currently used for the analysis of many different environmental and human matrices. One area of research where the use of NTS is in its early phase is for human biomonitoring. Target analysis of endocrine-disrupting chemicals (EDCs) in urine and serum has been applied since the early 2000s. Some of the prioritized EDCs have been phthalates and their metabolites, bisphenols, and parabens, while wide scope NTS analysis has been rare (7,8). In the study by Tkalec (8), an extraction method for human biomarkers in urine was developed and combined with LC–HRMS analysis and a suspect screening and NTS approach, and the workflow was validated by spiking known EDCs.
Another area of environmental NTS that is more developed is wastewater‑based epidemiology. It has been used to screen and prioritize potentially persistent, bioaccumulating, and toxic wastewater contaminants (9) and to investigate removal processes (9–12). The same has been done for advanced oxidation processes (AOP) of groundwater to prioritize compounds persistent to AOP processes, as well as TPs created by UV and hydrogen peroxide treatment that cannot be removed by active carbon filters (13).
Many more applications within environmental analysis have been proposed and tested, such as soil, sediment, and biota, showing the wide applicability of NTS for environmental analysis. Key developments are currently within analytical methods alternative to reversed-phase LC–HRMS for more water-soluble compounds (persistent mobile and toxic compounds [PMTs]) such as hydrophilic interaction liquid chromatography (HILIC), supercritical fluid chromatography (SFC), and ion and ion-pair chromatography (IC); workflows for more reliable peak detection, grouping of features, drift correction, and quantification without standards; and compound databases for precursors and TPs. The joint efforts by environmental analytical chemistry and data scientists worldwide have accelerated the development of a first guideline towards standardization of NTS in water analysis, which was published by the German Water Chemistry Society, “Non-Target Screening” Expert Committee (14).
References
1) L. Zimmermann, M. Scheringer, B. Geueke, J.M. Boucher, L.V. Parkinson, K.J. Groh, and J. Muncke, J. Hazard Mater.437, 129167 (2022).
2) K. Bhunia, S.S. Sablani, J. Tang, and B. Rasco, Compr. Rev. Food Sci. Food Saf. 12, 523–545 (2013).
3) F.S. Edaes and C.B. de Souza, Endocr. Metab. Immune Disord. Drug Targets 22, 927–934 (2022).
4) S. Tisler and J.H. Christensen, J. Hazard. Mater. 429, 128331 (2022).
5) S. Merel and S.A. Snyder, Environ. Int. 96, 98–117 (2016).
6) M.L. di Lorenzo and A. Longo, Thermochim. Acta 677, 180–185 (2019).
7) Ž. Tkalec, G. Codling, J.S. Tratnik, D. Mazej, J. Klánová, M. Horvat, and T. Kosjek, Environmental Pollution 313, 120091 (2022).
8) Ž. Tkalec, G. Codling, J. Klánová, M. Horvat, and T. Kosjek, Chemosphere 300, 134550 (2022).
9) K.M. Blum, P.L. Andersson, G. Renman, L. Ahrens, M. Gros, K. Wiberg, and P. Haglund, Science of the Total Environment 575, 265–275 (2017).
10) E.L. Schymanski et al., Analytical and Bioanalytical Chemistry 407, 6237–6255 (2015).
11) J.E. Schollée, M. Bourgin, U.V. Gunten, C.S. McArdell, and J. Hollender, Water Research 142, 267–278 (2018).
12) S. Tisler, N. Engler, M.B. Jørgensen, K. Kilpinen, G. Tomasi, and J.H. Christensen, Water Res. 219, 118599 (2022).
13) S. Tisler, P.L. Tüchsen, and J.H. Christensen, Environmental Pollution 309, 119758 (2022).
14) W. Schulz, T. Lucke, et al., Non-Target Screening in Water Analysis - Guideline for the application of LC-ESI-HRMS for screening (2019): Download at http://www.wasserchemische-gesellschaft.de
Selina Tisler is an assistant professor in the Analytical Chemistry Group at Department of Plant and Environmental Sciences, University of Copenhagen
Jan H. Christensen is a professor in the Analytical Chemistry Group at Department of Plant and Environmental Sciences, University of Copenhagen.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.