Separation Science: The State of the Art: The Future of Column Packing Technology
Columns packed with chemically optimized stationary phase particles are the workhorse of liquid chromatography (LC) analysis. Despite the decades of work spent in optimizing particle manufacturing and column packing procedures, we still lose about 50–60% of their separation potential to the random nature with which these particles are being packed. What if we could three-dimensionally (3D)-print these particles in ordered packing layers?
It is by now very well-known that the infamous eddy dispersion wastes more than half of the number of theoretical plates N that state‑of‑the‑art high performance liquid chromatography (HPLC) columns would be able to produce. This has been demonstrated in a number of computational studies studying the band broadening in ordered sphere packings, as these represent the ultimate degree of eddy dispersion elimination (1,2). Roughly speaking, reduced plate heights can be reduced from around h = 1.9–2 (fully particles) to around 0.8–1.0 near the optimum, while at 4× the optimal flow rate of the random packed bed gains already amount up to a factor of 3 to 4.
A first solution to produce chromatographic beds with a perfectly ordered structure are pillar-array columns (3). These are produced using state-of-the-art micromachining to produce silicon pillar arrays that are subsequently electrochemically anodized to produce a mesoporous outer shell. Because of their high efficiency, pillar-array columns have rapidly grown into the column of preference for many key players in the proteomics area (4,5). Limitations of the production method are the small scale of the produced micro‑arrays, typically only suitable for the nano-flow format, although recently some new designs targeting the micro- and capillary-flow regimes have been proposed. Because the etching process is unidirectional, only 2.5D-beds (pillar arrays) can be produced. This implies the top and bottom surfaces of the channel inevitably induce an extra velocity gradient, extending its effect across the entire channel depth. This in turn generates an additional degree of dispersion not present in 3D beds. In addition, and more importantly, the number of available stationary phases is limited, since pillar-array columns are currently a single-vendor product. This poses a considerable hurdle to their widespread use, as analysts—especially those in the pharmaceutical industry—have many good reasons to stick to the stationary phase and even the specific brand their original methods have been developed with.
The above implies there remains a strong impetus to produce ordered 3D beds, preferably making up a monolithic body of a suitable mesoporous material (silica, some polymer types). When contemplating the possibility of producing ordered 3D packings, the first technology that comes to mind is 3D-printing. However, most of the available 3D-printing methods (filament printing, stereolithography, digital light processing, inkjet/polyjet [i3DP] printers) do not offer the required ultra-small printing resolution (which is needed to produce a packing structure suitable for analytical chemistry, see below). Up to this point, 3D-printing in liquid chromatography has therefore been limited to printed devices targeting, at best, the preparative‑scale (6), where packing structure sizes in the 20 μm range are on the verge of being competitive. Other 3D-printing projects have been even more modest and only aimed at printing the exterior column housing (7). The only 3D-printing approach enabling the sub-micrometre printing resolution required for analytical liquid chromatography is two-photon polymerization (2PP) (8). As this approach requires the simultaneous absorption of two instead of only one photon to activate the photo‑initiator molecules, and, as the probability for such an event is only high enough in the central portion of the focal spot, resolutions below the wavelength of the light can be achieved. Using tailored monomer mixtures, voxel (=”volumetric pixel”) sizes as small as 200 nm can be printed using 2PP, which is small enough to print structures with 0.6 μm through-pores without suffering from excessive pore-to-pore shape and size differences. This pore size is comparable to the through-pore in a 2 μm particle-packed column, as is needed to be competitive with these columns. However, these very small voxel sizes inevitably also come at the expense of very low volumetric printing speeds. In a recent study (8), we showed the main drawback of this approach is that the printing process is inherently slow, requiring several 100’s of hours (!) to just print a structure with the dimensions of a typical nano-LC column (75 μm internal diameter [i.d.] cylindrical tube with length L = 15 cm). Another limitation is the available 2PP‑optimized printing resins. These are mostly of the acrylic type, and are hence difficult to derivatize, let alone amenable to forming a mesoporous structure. The ideal 3D-printed bed for liquid chromatography should be made of mesoporous silica, but the direct printing of ceramic structures is up to now limited to filament printers, which come nowhere close to the required resolution.
An ideal 3D-packed bed could be realized by developing a technology that can deposit entire layers of (chromatographic) spherical particles at the same time. This would speed up the printing process tremendously and produce structures made with suitable material. An approach to realize this (Figure 1) is under investigation in PrintPack, a research project funded via an Advanced ERC Grant. In its first realization (9), we managed to produce uniform monolayers using a two-step process. First, we assembled two-dimensional (2D)‑sphere arrays by suspending the particles in an electrostatic cell and simultaneously attracting them using a vacuum‑driven flow onto a silicon membrane perforated by an ordered array of through-pores and subsequently brushing away the inevitable excess particles in a single or double sweep with a dense brush while maintaining the vacuum to keep the bottom layer of particles in place (Figure 2). As can be noted from Figure 2, this approach allows error-less ordered sphere particle monolayers to be produced. In the next step, we could demonstrate that the monolayers can be flawlessly transferred onto a second substrate, provided this is made of an elastomeric material, for example, carbon tape or polydimethylsiloxane (PDMS). The project’s next phase will target contacting the layer of particles with a (sub-micrometre) thin layer of photocurable glue, and then deposit the particle layer onto a stack of previously deposited layers with the glue layer facing downwards (Figure 1[b–c]). Subsequent illumination with UV-light would then fix the particles in their position, followed by the release of the vacuum-assembly tool holding the particles during the assembly process. As already demonstrated, this can be realized by making use of the reversibility of the vacuum‑driven holding mechanism, that is, by applying an overpressure instead of an underpressure.
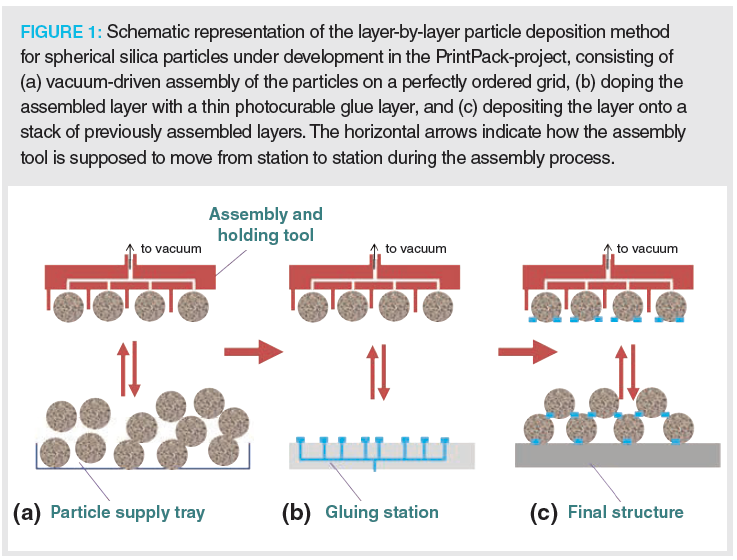
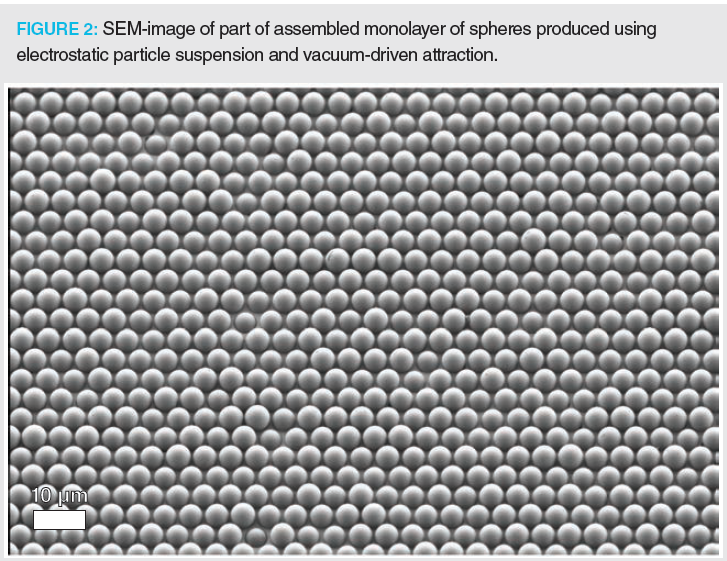
Although many hurdles are still to be overcome, for example, putting the produced arrays in perfect‑fitting and high-pressure‑resistant housing and finding ways to handle the polydispersity of some commercial HPLC particle types, it is believed this particle assembly and deposition process holds the promise that in the future one would be able to build perfectly ordered monolithic particle beds, offering twice the efficiency of our current randomly packed columns.
Acknowledgements
The funding from the ERC Advanced Grant “PrintPack” is gratefully acknowledged (No. 695067).
References
1) M.R. Schure, R.S. Maier, D.M. Kroll, and H.T. Davis, Anal. Chem. 74, 6006–16 (2002).
2) F. Mattheuse, S. Deridder, and G. Desmet, J. Chromatogr. A 1634, 461710 (2020).
3) W. De Malsche, H. Gardeniers, and G. Desmet, Anal. Chem. 80, 5391–400 (2008).
4) A. Mund, A.D. Brunner, and M. Mann, Mol. Cell. 82, 2335–2349 (2022).
5) J. Stadlmann, O. Hudecz, G. Krššáková, C. Ctortecka, G. Van Raemdonck, J. Op De Beeck, G. Desmet, J.M. Penninger, P. Jacobs, and K. Mechtler, Anal. Chem. 91, 14203–14207 (2019).
6) S. Nawada and T. Budel, LCGC Europe 34(9), 381–384 (2021).
7) [V. Gupta, S. Beirne, P.N. Nesterenko, and B. Paull, Anal. Chem. 90, 1186–1194 (2018).
8) F. Matheuse, K. Vanmol, J. Van Erps, W. De Malsche, H. Ottevaere, and G. Desmet, J. Chromatogr. A 1663, 462763 (2022).
9) W. Van Geite, I.S.M. Jimidar, K. Sotthewes, H. Gardeniers, and G. Desmet, Mater. Des. 216, 110573 (2022).
Gert Desmet is a full professor in the Department of Chemical Engineering, Vrije Universiteit Brussel, Belgium.
Ward Van Geite is a PhD researcher in the Department of Chemical Engineering, Vrije Universiteit Brussel.
Ignaas Jimidar is a post‑doc, Mesoscale Systems group, Twente University, The Netherlands/Department of Chemical Engineering, Vrije Universiteit Brussel.

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)