Separation Science: The State of the Art: New Frontiers in Multidimensional Liquid Chromatography
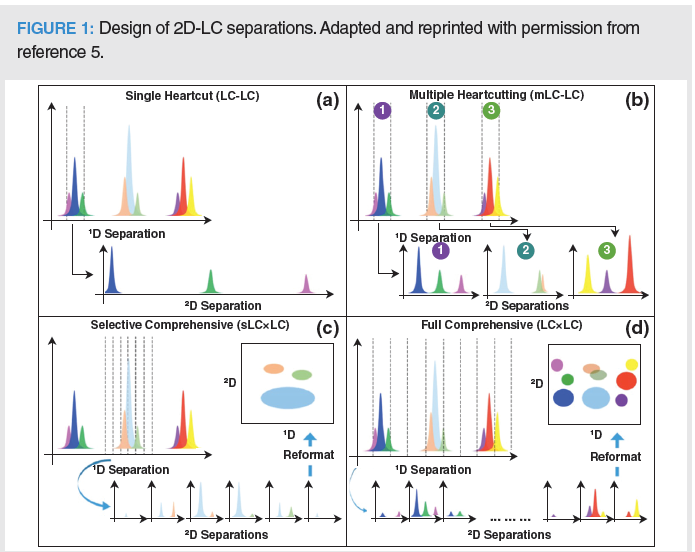
In recent years, multidimensional liquid chromatography (MD–LC) has become a very powerful analytical method for the successful analysis of nonvolatile analytes in complex real-world samples. MD–LC is a powerful alternative technique to conventional one-dimensional (1D)-LC and involves the use of two “orthogonal” liquid separation systems (1). The main advantage of such a technique is linked to the augmented separation power, as a consequence of the increased selectivity and sensitivity of the two systems. In MD–LC, the fractions eluting from the first dimension (1D) are fractionated and re-injected into the second dimension (2D). When only a few selected parts eluting from the 1D are analyzed in the 2D, the approach is regarded as “single” heart‑cutting (LC–LC; Figure 1[a]); when two or more selected fractions are diverted into the 2D, the technique is called “multiple heart-cutting” (mLC–LC; Figure 1[b]). In addition, if the whole sample is transferred to the 2D, a full “comprehensive” (LC×LC) approach is accomplished (Figure 1[d]). Last but not least, if a specific region of the 1D separation is considered for the analysis, a selective LC×LC (sLC×LC) is carried out (Figure 1[c]) (2). Clear advantages of the LC–LC and the LC×LC mode over the 1D-LC counterpart are increased orthogonality (A0) and peak capacity (nc), the possibility to test different retention mechanisms, and better compound organization due to the formation of structured 2D plots; the main disadvantages can be traced back to the instrumental complexity, reduced sensitivity, and need for fast and high flow to employ in 2D separations. Fraction transfer from the 1D to the 2D can be performed either in offline or online mode, in the latter case by the use of a modulator or an interface, which is considered the “heart” of the multidimensional approach. In offline mode, the fractions are manually collected, evaporated, and re-dissolved in a solvent compatible with the 2D mobile phase prior to being re-injected into the 2D. As a consequence, a series of 2D separations, related to the number of fractions from the 1D, are obtained. The simplicity of such an approach avoids the use of any sophisticated modulators or dedicated interfaces; alternatively, this method can bring sample loss or contamination and certainly tedious, time-consuming procedures (3–6). The online mode involves the use of switching valves equipped with either identical internal volume loops or trapping columns, with augmented reproducibility, reduced sample contamination, and less time‑consuming sample treatment steps.
As far as separation modes are concerned, in MD–LC, different retention mechanisms may be selected, for example, reversed-phase LC, normal‑phase LC, hydrophilic interaction LC (HILIC), ion-pairing chromatography (IPC), ion exchange chromatography (IEC), hydrophobic interaction chromatography (HIC). To achieve the best system “orthogonality”, the two dimensions must have a different and independent separation mechanism, which leads to enhanced peak capacity (7). Also, when the LC×LC technique is employed for high-resolution separations, a remarkable advantage over the 1D-LC is the rapid peak‑production rate, which is roughly 1 peak per second vs. 1 peak per minute for a conventional 1D-LC (7).
The capability of the MD–LC technique may be augmented if an MS system is connected to the 2D separations. In fact, this in turn permits unambiguous compound identification in the investigated sample and beneficially allows matrix effects that hamper both quali-quantitative results to be reduced. Enhanced separations and a decrease of sample complexity prior to MS detection has the advantage of minimizing ion suppression and improving ionization efficiency (8).
However, MD–LC does present some pitfalls, for example, length of analysis time, which is normally higher than 1D-LC, and complex equipment with specific data treatment software. Notably, potential immiscibility or incompatibility of the mobile phases in both dimensions have to be considered; in this context, newly developed approaches, for example, fixed solvent modulation (FSM) or active solvent modulation (ASM) have been proposed (9). In this configuration, the implementation of any valve. for example, a 10-port/2- position or 8-port/2-position, normally used for LC×LC, is augmented with a “bypass connector” that establishes a fluidic connection between the 2D pump and 2D column in parallel with the path through the valve. One significant limitation of the FSM approach is that the bypass connection is fixed, and flow will go through both the valve and the bypass connector throughout the analysis. Since the total flow from the 2D pump is split into two paths, the “actual” flow rate through the sample loop is lower than the total flow and dependent on the split ratio. The ASM approach focuses on a new valve-based approach to apply fraction transfer in LC×LC that enables effective focusing of analyte bands at the inlet of the 2D column without the need for any additional instrument hardware. Such an approach offers important advantages over FSM for effective analyte focusing at the 2D column inlet and can be summarized as: 1) More rapid flushing of high solvent strength eluent from the sampling loops at the end of each 2D separation cycle 2) Less complex mobile phase profiles delivered to the 2D column during gradient elution, which results in less baseline disturbances and a larger practical elution window in each 2D separation.
On the other hand, a practical limitation to the ASM approach is the time it takes to displace the fractions of effluent collected from the 1D column from the sampling loop into the 2D column. Apart from this, it is reasonable to believe the development of ASM represents a significant step forwards in terms of improving the ease-of-use of LC×LC and the overall detection sensitivity of the technique.
On the basis of the above considerations, it is predictable that MD–LC techniques unquestionably present fundamental advancements in terms of increased orthogonality, separation power, and sensitivity—even more enhanced if powerful MS detectors are hyphenated— thus leading to an ever increasing number of applications in several research fields.
References
1) D.R. Stoll, X. Wang, and P.W. Carr, Anal. Chem. 80, 268–278 (2008).
2) D.R. Stoll, K. Zhang, G.O. Staples, and A. Beck, Advances in Chromatography (CRC Press, Boca Raton, Florida, USA, 2018, Vol. 56).
3) F. Cacciola, P. Donato, D. Sciarrone, P. Dugo, and L. Mondello, Anal. Chem. 89, 414–429 (2017).
4) F. Cacciola, F. Rigano, P. Dugo, and L. Mondello, TrAC-Trend. Anal. Chem. 127, 115894 (2020).
5) K. Arena, F. Mandolfino, F. Cacciola, P. Dugo, and L. Mondello, J. Sep. Sci. 44, 17–34 (2021).
6) F. Cacciola, K. Arena, F. Mandolfino, D. Donnarumma, P. Dugo, and L. Mondello, J. Chromatogr. A 1645, 462129 (2021).
7) B.W.J. Pirok, D.R. Stoll, and P.J. Schoenmakers, Anal. Chem. 91, 240–263 (2019).
8) K. Arena, F. Cacciola, F. Rigano, P. Dugo, and L. Mondello, J. Sep. Sci. 43, 1781–1789 (2020).
9) D.R. Stoll, K. Shoykhet, P. Petersson, and S. Buckenmaier, Anal. Chem. 98, 9260–9267 (2017).
Francesco Cacciola is associate professor of food chemistry at the Dipartimento di “Scienze Biomediche, Odontoiatriche e delle Immagini Morfologiche e Funzionali” of the University Messina, Italy.
Katia Arena is assistant professor of food chemistry at the Dipartimento di “Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali” of the University Messina, Italy
Paola Dugo is full professor of food chemistry at the Dipartimento di “Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali” of the University Messina, Italy.
Luigi Mondello is full professor of analytical chemistry at the Dipartimento di “Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali” of the University Messina, Italy.

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)