Separation Science: The State of the Art: Analytical Chemistry: There is No Green Like More Green
Analytical chemistry is an important tier of environmental protection and has been traditionally linked to the assessment of the environmental quality status of systems. Although essential, analytical chemistry may also contribute to further environmental problems mainly due to the high energy demands and large quantities of hazardous substances that may be used or generated throughout an analytical procedure. These two distinct and contradictory roles of analytical chemistry were highlighted by Paul Anastas a year after the introduction of green chemistry (1), when the concept of green analytical chemistry (GAC) was defined as an emerging area, relevant to the research arena and commercial sector. At that time, traditional sample preparation methods were identified as a major source of the total negative impact of analytical methodologies on the environment and their replacement with contemporary ones was considered central in settling greener analytical methods. Indeed, early sample preparation methodologies were tedious, time-consuming, and, more importantly, expended large quantities of resources that resulted in the generation of hazardous laboratory waste.
In 2013, the concept of GAC was formulated in the form of 12 principles that expressed the willingness to care for the environment and human safety as part of the development and application of analytical procedures (2). The introduction of GAC aimed to redefine and reevaluate analytical methods by addressing safety of solvents/reagents, toxic laboratory waste generation, workers’ safety, and energy efficiency. In the formulation of GAC, the first principle suggested applying direct analytical techniques to avoid sample preparation and also concluded that any “green” action taken during the sample preparation step (for example, minimal use of energy, safety for operator, use of non-toxic reagents or reagents from renewable source) would have a negative impact on accuracy, precision, selectivity, sensitivity, and detectability of the analytical process. This was not a well‑reflected assumption, especially when considering the analytical performance of mature and green sample preparation technologies available at that time (for example, the solventless and reagentless solid-phase microextraction [SPME]). The use of direct analysis may be a straightforward approach to address problems related to sample preparation. However, to meet sensitivity needs and overcome matrix-related problems, the use of sophisticated, expensive, and energy‑consuming instrumentation is required that generally shifts the environmental impact from sample preparation to the determination step. More importantly, direct analysis is not always an option and a step of sample cleanup, analyte enrichment, or analyte conversion into a form suitable for analysis is commonly needed. In other words, sample preparation remains a key step in analytical procedures.
The first principle of GAC was commonly misinterpreted and created the false impression that omitting the sample preparation step is a green approach, fully neglecting the “green” technological advances in the field. The “exclusion” of sample preparation from GAC also created a gap by not considering cases where direct analysis was not an option. Instead of neglecting this step, efforts should have been devoted to fully defining sample preparation within the context of green chemistry and GAC. Over the years, redefining sample preparation to address sustainability issues and promote the practice of green sample preparation (GSP) has become a necessity. After all, green chemistry was never about what to stop doing, but was always about invention and the things one can do better.
Earlier this year, the concept of GSP was proposed (3). It was formulated in the form of 10 principles that represented a road map towards the development of overall greener analytical methodologies (Figure 1). This set of principles represented the optimum number of characteristics needed to describe the inner structure of the GSP concept, its properties, and function mechanism. The 10 principles were not isolated but formed an integrated system of design (Figure 1), where improvements achieved by aligning to the fulfilment of a given principle could synergistically help to reduce the deficiencies associated with other interconnected principles. GSP set goals that were common to GAC but also had several distinctive and innovative features. In every case, the GSP approach put sample preparation at centre stage and translated greenness based on the needs and requirements of sample preparation.
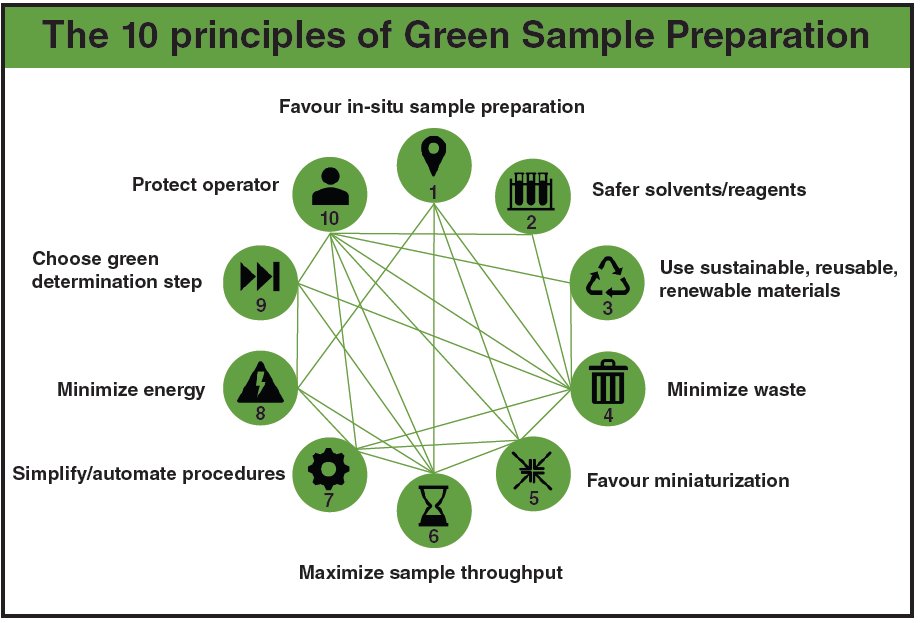
The aspects considered by GSP included the use of safe solvents/ reagents; materials being reusable and from renewable, recycled sources, minimizing waste generation and energy demand; minimization of samples, chemicals, and materials; procedure simplification and automation; operator’s safety; and preparing a high number of samples per unit time. Based on the advances in sample preparation, several contemporary and mature sample preparation technologies align with the principles of GSP and fulfil the requirements for greening this key step in analysis. It is equally important to note that the adoption of these methods in the laboratory not only aligns with the principles of GSP but also assists in improving the analytical characteristics of the overall method. The latter contrasts with the GAC approach that faces the challenge of reducing the environmental impact of methods without negatively impacting the analytical efficiency of the method, that is, sensitivity, selectivity, accuracy, precision, robustness, and, in turn, the quality of the analytical information obtained.
Current environmental challenges are of a global scale, highlighting the urgent need to align with pollution abatement and the principles of sustainable development. Analytical chemists face increasingly complex interrelated problems both on-site and at the laboratory and the application of environmentally benign analytical practices has become a critical factor to consider. The adoption of contemporary sample preparation practices with a low environmental impact should therefore be a priority for all researchers, practitioners, and routine analysts. Greening analytical methods is directly connected to greening the sample preparation step. In other words, there is no green like more green.
Acknowledgements
This article is based upon work from the IUPAC project No 2021-015-2- 500 “Greenness of official standard sample preparation methods” and work from the Sample Preparation Study Group and Network, supported by the Division of Analytical Chemistry of the European Chemical Society.
References
1) P.T. Anastas, Crit. Rev. Anal. Chem. 29, 167–175 (1999).
2) A. Gałuszka, Z. Migaszewski, and J. Namieśnik, TrAC - Trends Anal. Chem. 50, 78–84 (2013).
3) Á.I. López-Lorente, F. Pena-Pereira, S. Pedersen- Bjergaard, V.G. Zuin, S.A. Ozkan, and E. Psillakis, TrAC Trends Anal. Chem. 148, 116530 (2022).
Elia Psillakis is full professor in water chemistry at the School of Chemical and Environmental Engineering, Technical University of Crete, Greece.
Stig Pedersen-Bjergaard is a professor in the Department of Pharmacy, University of Oslo, Norway, and Department of Pharmacy, University of Copenhagen, Denmark.
Sibel A. Ozkan is a full professor of analytical chemistry at Ankara University, Faculty of Pharmacy, Department of Analytical Chemistry in Turkey.

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)