Improved Analysis of Preservatives in Cosmetics Using a Unique C18 Core-Shell Phase
The Application Notebook
This study evaluates the performance of a C18 core-shell phase that incorporates a C18 ligand with iso-butyl side chains. The Kinetex? XB-C18 HPLC/ UHPLC column delivers a fast and effective separation of several common preservatives in cosmetics.
This study evaluates the performance of a C18 core-shell phase that incorporates a C18 ligand with iso-butyl side chains. The Kinetex® XB-C18 HPLC/ UHPLC column delivers a fast and effective separation of several common preservatives in cosmetics.
Preservatives prevent product deterioration and deter any possible health risks microorganisms may cause to the consumer; however, a disadvantage of using such agents is that they may cause adverse effects, such as allergic responses and irritation. There is a need today for a fast, efficient, and selective HPLC method to screen for such common and dangerous preservatives.
Experimental Conditions
Analyses performed using an HP 1100 LC system (Agilent Technologies, Palo Alto, California) with an upper pressure limit of 400 bar, equipped with a UV detector.
Column: Kinetex 2.6 μm XB-C18 100 Å
Dimensions: 100 × 4.6 mm
Mobile Phase: A: Water with 0.1 % TFA
B: Acetonitrile with 0.1 % TFA
Gradient: (85:15) A/B for 20 min, then to (15:85) A/B
Flow Rate: 1.5 mL/min
Column Temperature: 30 °C
Detection: UV @ 214 mm (ambient)
Injection Concentration: 50 μg/mL
Sample: 1. Benzyl alcohol; 2. Phenoxyethanol; 3. Sorbic acid; 4. Benzoic acid; 5. Methyl paraben; 6. p-Anisic acid; 7. Dehydroacetic acid; 8. Salicylic acid; 9. Ethyl paraben; 10. Isopropyl paraben; 11. Propyl paraben; 12. Isobutyl paraben; 13. Butyl paraben; 14. Triclosan; 15. Triclocarban
Results
Cosmetic products can only use a limited number of preservatives selected from a positive list, Annex VI of the Cosmetics Directive, which also defines preservative maximum permitted levels and areas of use. The esters of parahydroxybenzoic acid (paraben), methyl paraben, ethyl paraben, propyl paraben, and butyl paraben are standard substances among the preservatives list.
Figure 1 illustrates the ability of the Kinetex XB-C18, 2.6 μm core-shell column to rapidly screen and separate all 15 compounds. In this separation, the Kinetex XB-C18 column offered a peak capacity of 445, which was higher than any other column evaluated in the experiment. Peak capacity is the best measure of performance for a gradient separation and high peak capacity values indicate increased analyte resolution over a given analysis time.
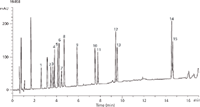
Figure 1: High performance separation of 15 preservatives on Kinetex 2.6 μm XB-C18.
The core-shell particle morphology allows for faster mass transfer of analytes into and out of the stationary phase as compared to fully-porous silica particles. In addition, the very narrow particle size distribution inherent in core-shell silica particles, as compared to fully-porous particles, results in less band broadening.
Kinetex 2.6 μm XB-C18 was able to separate the chlorinated compounds triclosan and triclocarban, which can be challenging. This is likely due to the unique XB-C18 selectivity. The Kinetex XB-C18 chemistry contains protective di-isobutyl side chains that shield the silica surface. In addition, the surface is endcapped with trimethylsilane.
Analysis of preservatives in cosmetics was accomplished at an operating pressure under 400 bar and may therefore be used on conventional HPLC systems without the need for specialized ultra-high pressure equipment.
Conclusion
An ultra-high performance liquid chromatography method has been developed for the simultaneous determination of 15 preservatives in cosmetics. The method was developed to achieve the best balance of analysis time and separation.
The Kinetex XB-C18, 2.6 μm column provided high peak capacity and the unique selectivity of the XB-C18 was well-suited for the application.
Phenomenex, Inc.
411Madrid Avenue, Torrance, CA 90501
tel. (310) 212-0555, fax (310) 328-7768
Website: www.phenomenex.com

Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
Using GC-MS to Measure Improvement Efforts to TNT-Contaminated Soil
April 29th 2025Researchers developing a plant microbial consortium that can repair in-situ high concentration TNT (1434 mg/kg) contaminated soil, as well as overcome the limitations of previous studies that only focused on simulated pollution, used untargeted metabolone gas chromatography-mass spectrometry (GC-MS) to measure their success.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.
Potential Obstacles in Chromatographic Analyses Distinguishing Marijuana from Hemp
April 28th 2025LCGC International's April series for National Cannabis Awareness Month concludes with a discussion with Walter B. Wilson from the National Institute of Standard and Technology’s (NIST’s) Chemical Sciences Division regarding recent research his team conducted investigating chromatographic interferences that can potentially inflate the levels of Δ9-THC in Cannabis sativa plant samples, and possible solutions to avoid this problem.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)



















