Determination of Phthalate Esters in Child Care Products and Children's Toys by Gas Chromatography–Mass Spectrometry (GC–MS)
The Application Notebook
The Consumer Product Safety Improvement Act of 2008 (CPSIA) requires testing of child care products and toys for selected phthalate esters by GC–MS.
The Consumer Product Safety Improvement Act of 2008 (CPSIA) requires testing of child care products and toys for selected phthalate esters by GC–MS. The CPSC test method specifies GC–MS analysis in the SIM mode to monitor for low-intensity ions that are unique to specific phthalate esters, but full-scan mass spectra are valuable in qualitative identification. Operation of the mass spectrometer in the FASST Scan/SIM mode allows concurrent acquisition of full-scan and SIM mass spectral data, to provide improved qualitative identification while still maintaining optimum sensitivity.
Two of the regulated compounds are mixtures of isomers, and quantitation ions for these compounds are of very low relative intensity. So trace detection for these two substances is more challenging than for the other regulated compounds.
Terephthalate esters (non-regulated isomers of phthalate esters) are frequently present at high concentration in real-world samples and interfere with identification and quantitation of several of the regulated phthalate esters. Analysis on an alternate column provides chromatographic resolution of interferences from the regulated phthalate esters.
Results
Analyses were conducted using a Shimadzu GCMS-QP2010S operated in the FASST (Scan/SIM) mode. Two chromatographic columns (0.25 mm × 30 m × 0.25 μm) were employed: RXI-5MS and RXI-17Sil MS (Restek Corporation). The following instrument conditions were employed for the analyses:
GC Conditions
- Injector Temp: 330 °C
- Injection Mode: Splitless (1 min)
- Carrier: He; Constant Linear Velocity (36 cm/s)
- Column Temperature Program: 50 °C (hold 1 min), program 30 °C/min to 280 °C, then 15 °C/min to 330 °C (hold 3 min)
MS Conditions
- Interface Temp: 330 °C
- Ion Source Temp: 255 °C
- Data Acquisition: FASST Scan/SIM Mode; Scan 50–500 0.15 s, SIM (group) 0.10 s
A chromatogram of the regulated phthalate esters on an RXI-5MS column is shown in Figure 1. The traces for diisononyl phthalate and diisodecyl phthalate on the chromatogram are mass chromatograms for the quantitation ions m/z 293 and 307, respectively. Under these conditions, di-n-octyl terephthalate (DOTP) coelutes with di-n-octyl phthalate.
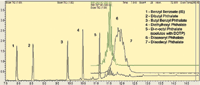
Figure 1: Chromatogram of phthalate esters on RXI-5MS column.
A chromatogram of the regulated phthalate esters on an RXI-17SilMS column is shown in Figure 2. Under these conditions, DOTP is well resolved from the regulated phthalate esters. The chromatographic separation of the phthalate esters is also very good on the RXI-17SilMS column, but elution of butyl benzyl phthalate is much later on this column.
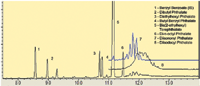
Figure 2: Chromatogram of phthalate esters on RXI-17 SilMS column.
Conclusion
In summary, qualitative identification is improved and excellent sensitivity is maintained for this method using FASST Scan/SIM, relative to SIM (only) data acquisition. One common chromatographic interference on the method-specified chromatographic column was resolved from the target analytes using an alternate column.
Shimadzu Scientific Instruments
7102 Riverwood Drive, Columbia, MD 21046
tel. (800) 477-1227, fax (410) 381-1222
Website: www.ssi.shimadzu.com

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
Investigating the Protective Effects of Frankincense Oil on Wound Healing with GC–MS
April 2nd 2025Frankincense essential oil is known for its anti-inflammatory, antioxidant, and therapeutic properties. A recent study investigated the protective effects of the oil in an excision wound model in rats, focusing on oxidative stress reduction, inflammatory cytokine modulation, and caspase-3 regulation; chemical composition of the oil was analyzed using gas chromatography–mass spectrometry (GC–MS).



















