Analyzing Testosterone in Human Serum by UHPLC Using High Efficiency Kinetex 1.7 m C18 Core-Shell Columns
The Application Notebook
Testosterone was extracted from human serum by strong anion exchange polymeric SPE and analyzed using a Kinetex C18, 30 ? 2.1 mm, 1.7 ?m column and positive polarity ESI LC–MS-MS system. Kinetex sub-2 ?m core-shell technology offers higher efficiencies than traditional sub-2 ?m columns, producing greater chromatographic resolution, sensitivity, and higher peak capacities.
Seyed Sadjadi and Jeff Layne*, Phenomenex, Inc.
Testosterone was extracted from human serum by strong anion exchange polymeric SPE and analyzed using a Kinetex C18, 30 × 2.1 mm, 1.7 µm column and positive polarity ESI LC–MS-MS system. Kinetex sub-2 µm core-shell technology offers higher efficiencies than traditional sub-2 µm columns, producing greater chromatographic resolution, sensitivity, and higher peak capacities.
Testosterone is an androgenic steroid responsible for the development of male reproductive organs, maintaining (or increasing) muscle mass and bone density. As anabolic steroids, testosterone has been used (or abused) to increase muscle mass and enhance the athletic performance. The concentration of testosterone is lower in the female population than the male and in general diminishes with advancing age. Monitoring body concentration of testosterone is an aid in diagnosing and treating disease state related to the hormonal imbalance.
The analysis is based on a simple extraction method using strong anion exchange SPE (Strata-X-A) to produce a clean extract from human serum. Following the extraction, testosterone is derivatized to form an oxime which is then analyzed in positive mode ESI LC–MS-MS under multiple-reactions-monitoring function (1). A short-length 30 mm, 1.7 um Kinetex C18 column efficiently separates testosterone from its isomeric form epitestosterone (Figure 1).

Figure 1: The separation of 100 pg/mL standard of testosterone and epitestosterone extracted from human serum on Kinetex C18, 30 Ã 2.1 mm, 1.7 um using the LC gradient profile listed in Table I. Testosterone retention time is 2.62 min, epitestosterone 2.77 min, and int. std. is 2.61 min.
Experimental Conditions
The mobile phase consisted of 0.1% formic acid with 1 mM ammonium formate with no pH adjustment, in water (MP A) and acetonitrile (MP B). A typical LC gradient is used (Table I) for the separation.
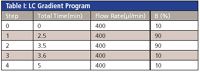
Table I: LC Gradient Program
An AB Sciex API 5000 triple-quadrupole tandem mass spectrometer is used for analysis equipped with an ESI probe operating in positive polarity mode. Under an MRM mode, two channels were monitored for testosterone and testosterone-D3 (Table II).
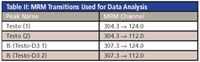
Table II: MRM Transitions Used for Data Analysis
Results and Discussions
As is demonstrated from the chromatogram, the Kinetex column provides a high degree of selectivity even in small dimensions to provide superior chromatographic separation. For further details or questions, contact your Phenomenex sales representative.
References
(1) M.M. Kushnir et al, Clinical Chemistry 52:1, 120–128 (2006).
Phenomenex, Inc.
411 Madrid Avenue, Torrance, CA 90501
tel. (310) 212-0555, fax (310) 328-7768
Website: www.phenomenex.com

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)



















