USP <467> Headspace Residual Solvent Assay with a HT3 Headspace Instrument
The US Pharmacopeia recently released USP<467> as the current monograph for determining residual solvents in pharmaceutical products by static headspace.
Roger Bardsley, Teledyne Tekmar
The US Pharmacopeia recently released USP<467> as the current monograph for determining residual solvents in pharmaceutical products by static headspace. Extensive method validation is not required if the USP parameters are followed. Class 1 and Class 2 residual solvents are determined by the headspace method.
The Class 1 compounds were evaluated from 50% to 500% of the USP limits, while the Class 2 compounds were evaluated from 25% to 400% of the USP limits.
Experimental-Instrument Conditions
The HT3™ headspace instrument sample temperature was set to 80 °C for 45 min in the static mode. The valve oven and transfer line temperature were set to 110 °C as allowed by the USP. A Phenomenex ZB-624 column, 30 m × 0.32 mm × 1.8 µm was selected for the G43 column. A Shimadzu GC-21010 GC/FID was programmed using the GC parameters from the USP method.
Standard Sample Preparation
Class 1, Class 2A, and Class 2B standard stock solutions were prepared following the USP <467> procedure. Analyzing the standards in triplicate demonstrates the compounds reproducibility.
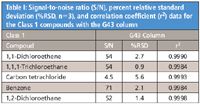
Table I: Signal-to-noise ratio (S/N), percent relative standard deviation (%RSD, n=3), and correlation coefficient (r2) data for the Class 1 compounds with the G43 column
A series of standards were prepared from 50% to 500% of the concentration of the Class 1 Standard. Similar standards from 25% to 400% were generated for the Class 2A and 2B standards. Individually analyzing these samples verified the linearity of the method over these ranges.

Table II: Resolution (R), percent relative standard deviation (%RSD, n=3), and correlation coefficient (r2) data for the Class 2A compounds with the G43 column
Results
Results for the G43 and the G16 columns are presented in Table I for the Class 1 compounds, Table II for the Class 2A compounds, and Table III for the Class 2B compounds.
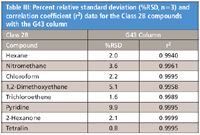
Table III: Percent relative standard deviation (%RSD, n=3) and correlation coefficient (r2) data for the Class 2B compounds with the G43 column
Conclusions
Using a Teledyne Tekmar HT3 headspace instrument with Phenomenex columns and a Shimadzu GC/FID adequately meets the requirements of the recently released USP Chemical Test Monograph <467> for residual solvents.
Teledyne Tekmar
4736 Socialville Foster Road, Mason, OH 45040
tel. (800) 874-2004, fax (531) 229-7050
Website: www.teledynetekmar.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














