Ultra High Throughput Analysis of Basic Drugs and Steroids Utilizing New 3 µm Column Technology
The demand for faster throughput in LC–MS drug discovery labs has increased throughout the years.
The demand for faster throughput in LC–MS drug discovery labs has increased throughout the years. New technologies from both instrument and column manufacturers have been designed in order to meet this demand. In particular, the combination of UHPLC systems with 2 µm particle columns has been advertised as a possible solution. In this paper, we present an alternative solution using 3 µm particle columns. The premise is that observed performance gains are remarkably small when transitioning from 3 to 2 µm particles, while the pressure increases are large, which limits maximum eluent velocity through the column and hence analysis speed. Instead of using smaller particle diameter to increase optimum velocity, we use elevated temperature, which allows us to achieve and optimize 10 mm/s eluent velocities instead of the practical upper limit of 5 mm/s encountered with 2 µm and smaller particles.

Figure 1: Basic drugs.
Experimental
All data were generated with a UHPLC system, equipped with Cadenza CD-C18 HT (3 µm particle) column. This new column design has a 50 MPa max pressure limit, which is significantly larger than the pressure limit for conventional HPLC columns (~20 MPa).
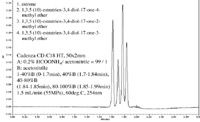
Figure 2: Estrogens.
Results and Discussion
By using a low dispersion instrument with high pressure capabilities (e.g., UHPLC), we have shown that ultra high throughput analysis, with similar or the same performance as 2 µm particle columns, can be achieved on 3 µm columns (2 min run times). This decreased resistance to flow, which results from the use of a larger 3 µm particle column and elevated temperature, allows for higher k' per unit time and thus higher productivity. As a result, it is possible to achieve higher eluent velocities (10 mm/s instead of 5 mm/s) and thus faster throughput with 3 µm particle columns than with 2 µm particle columns. This facilitates highly selective analysis of pharmaceuticals and biomarkers at unprecedented speeds as shown in Figures 1–3.

Figure 3: Steroid hormones.
References
(1) M.J. Hayward, M.D. Bacolod, Q.P. Han, M. Cajina, and Z. Zou, Chapter 11 — Techniques to Facilitate the Performance of Mass Spectrometry: Sample Preparation, Liquid Chromatography, and Non-Mass-Spectrometric Detection in "Mass Spectrometry in Drug Metabolism," M. Lee, M. Zhu, ed., Wiley, New York: 2010.
Imtakt USA
1511 Walnut St., Suite 310, Philadelphia, PA 19102
tel. (888) 456-HPLC, (215) 665-8902, fax (501) 646-3497
Email: info@imtaktusa.com, Website: www.imtaktusa.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















