Ethanolamines in Water Samples by LC–MS
This rapid LC–MS method shows sensitive and selective separation of five ethanolamine analytes in wastewater.
Leo (Jinyuan) Wang and William C. Schnute, Dionex Corporation
This rapid LC–MS method shows sensitive and selective separation of five ethanolamine analytes in wastewater.
Ethanolamines have been used as bio- and environmental markers for nitrogen mustards to measure potential exposures because direct quantification of nitrogen mustards is difficult due to their instability (1). Ethanolamines are also manufactured in large volume (over half a million tons per year) for a wide range of industrial and domestic uses such as in the manufacture of pesticides, emulsifying agents and detergents, and bactericides and cosmetics (2). To monitor the removal of ethanolamines from industrial discharged wastewater and the extent of human and environmental exposure to nitrogen mustards, a quantitative analytical method is desired.
An LC–MS method is described here using a mixed-mode column featuring reversed phase and cation exchange retention mechanisms providing sufficient retention and resolution for all analytes in 6 min. The MSQ Plus™ mass spectrometric detector was operated in selected ion monitoring (SIM) mode to ensure sensitive and selective sub-ppb level detection.
Experimental
This study was performed on a Dionex UltiMate® 3000 LC system. The separation was achieved on an Acclaim® Trinity™ P1 column (2.1 × 100 mm, 3 µm) operated at 20 °C. The isocratic mobile phase used for the separation was composed of 90% CH3CN, 7% DI water, and 3% ammonium formate buffer (pH 3.7, 100 mM) with a flow rate of 0.5 mL/min. A 20 µL aliquot of each sample was injected for analysis.
An electrospray ionization (ESI) source was used to couple the LC–MS system, with the needle voltage set at 1000 V and probe temperature set at 500 °C. Nitrogen was used as the nebulizer gas at 80 psi. The MSQ Plus system was operated in positive SIM mode with optimized collision voltages for each of the analyte m/z values: ethanolamine (EA): 62 m/z, 30 V; diethanolamine (DEA): 106 m/z, 40 V; diethanolamine-d8 (DEA-IS): 114 m/z, 40 V; N-methyldiethanolamine (MDEA): 120 m/z, 30 V; N-ethyldiethanolamine (EDEA): 134 m/z, 35V; triethanolamine (TEA): 150 m/z, 45 V.
Results and Conclusions
As seen in Figure 1, the five target ethanolamines were retained and separated on the Trinity column within 5 min and selectively detected using SIM scans. Sub-ppb analyte levels can be routinely quantified using this method, with Figure 1 showing the chromatograms of a standard containing 1 ppb of each analyte. Excellent coefficient of determination was achieved with R2 greater than 0.99 for each analyte from its detection limit to 100 ppb. Detection limits were defined as the lowest concentration of calibration standard showing a signal-to-noise ratio greater than 5 (1 ppb for ethanolamine, and 0.1 ppb for all other target analytes).
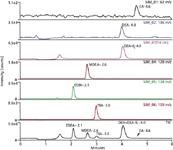
Figure 1: Analysis of five target ethanolamines (1.0 ppb each) by LCâMS.
References
(1) R.M. Black and R.W. J. Read, Chromatogr. 449, 261–270 (1998).
(2) C. Edser, Focus on Surfactants7, 1–2 (2004).
UltiMate and Acclaim are registered trademarks, and Trinity is a trademark of Dionex. MSQ Plus is a trademark of Thermo Fisher Scientific.
Dionex Corporation
1228 Titan Way, P.O. Box 3603, Sunnyvale, CA 94088
tel. (408) 737-0700, fax (408) 730-9403
Website: www.dionex.com

Separation of Ultra-Short and Long Chain PFAS Compounds Using a Positive Charge Surface Column
December 11th 2024A separation of ultra-short and long chain PFAS (C1-C18) is performed on a HALO®PCS Phenyl-Hexyl column along with a HALO®PFAS Delay column which demonstrates excellent retention for both hydrophilic and hydrophobic analytes.















