Determination of Phospholipids by HPTLC
Phospholipids are important compounds for the formation of bilayer membranes in biological systems.
Phospholipids are important compounds for the formation of bilayer membranes in biological systems. Forming vesicles like liposomes or micelles charged with active ingredients or used as basic mixtures for building emulsions they are of high interest to the pharmaceutical and cosmetic industry. Quantitative determination of phospholipids is also important for the food industry with respect to declaration of prophylactic and therapeutic effects of dietary phospholipids.
This method is suitable for screening and quantification of phospholipids like lysophophatidic acid (LPA), lysophosphatidyl-choline (LPC), lysophosphatidylethanolamine (LPE), lysophosphatidylinositol (LPI), phosphatic acid (PA), phosphatidyl-choline (PC), phosphatidyl-ethanolamine (PE), and phosphatidyl-inositol (PI).
Required or Recommended CAMAG devices
Automatic TLC Sampler 4 or Linomat 5
Automatic Developing Chamber ADC2 with humidity control 47% r.H.
Immersion Device III, Plate heater
TLC Scanner 4 and winCATS software or Visualizer and VideoScan software
Derivatization Reagent
Copper(II) sulfate: Dissolve 20 g of copper sulfate pentahydrate in 200 mL of methanol at less than 20 °C. Under cooling with ice add 8 mL of sulfuric acid 98% and 8 mL of ortho-phosphoric acid 85%.

Figure 1: Separation of eight phospholipids on HPTLC plates.
Sample
Examples given: samples from skin (see application note A-89 or A-90).
Standards
A standard solution containing 0.1 mg/mL of phospholipids in chloroform, methanol 2:1.
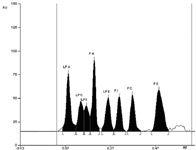
Figure 2: Densitogram of eight phospholipids.
Chromatography
Stationary phase: HPTLC Si 60 F254 20 × 10 cm (Merck)
Sample application: 2–10 µL of standard are applied as 8 mm bands, min 2 mm apart, 8 mm from lower edge of plate
Developing solvent: Chloroform, methanol, water, ammonia 25% (60:34:4:2)
Development: ADC2 with humidity control at 47% relative humidity using a saturated KSCN solution, chamber saturated for 20 min (using filter paper), 10 mL developing solvent per trough.

Figure 3: Calibration fuction for phosphatidly-choline (PC).
Developing distance: 60 mm from lower edge of plate
Plate drying: 5 min in a stream of cold air
Derivatization: Copper(II) sulfate: immerse the plate into the reagent for 6 s. Dry the plate during 30 s with cold air and heat it at 140 °C for 30 min using a plate heater.
Evaluation: Examination in white light
Densitometry
With CAMAG TLC Scanner 4 and win CATS software in absorption mode at 360 nm using a deuterium lamp, 420 or 720 nm using a tungsten lamp. Alternatively video densitometry (ViedoScan) of images taken under white light. Evaluation via peak height or area, polynomial regression.
CAMAG Laboratory
Sonnenmattstrasse 11, P.O. Box 216
CH-4132 Muttenz 1, Switzerland
tel. +41-61-467 34 34, fax +41-61-461 07 02
Email: lab@camag.com, Website: www.camag.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















