Determination of Free and Total Glycerol in Biodiesel
Biodiesel fuels are part of a rapidly growing alternative fuel industry to replace gasoline with renewable, more efficient, and lower emission fuels (1).
Terri T. Christison, Brian M. De Borba, and Jeffrey S. Rohrer, Dionex Corporation
Biodiesel fuels are part of a rapidly growing alternative fuel industry to replace gasoline with renewable, more efficient, and lower emission fuels (1). Biodiesel contains diesel fuel blended with 2–20% (B2–B20) of fatty acid methyl esters (FAME), which are manufactured by transesterification of vegetable or animal oils to generate three molecules of free FAMEs and one molecule of glycerol by-product. Free and bound glycerol from unconverted triglycerides can cause diesel engine failures. Therefore, ASTM D6751 specifies limits for free glycerol and bound glycerol at 0.02 and 0.24 wt%, respectively in biodiesel determined using GC-FID (1). However, this method requires sample derivatization, which increases sample handling time and expense. This application note describes a high-performance anion-exchange chromatography with pulsed amperometric detection (HPAE-PAD) method for free and total glycerol in biodiesel that meets the ASTM limit requirements and eliminates the sample derivatization step.
Experimental
A Dionex ICS-3000 chromatography system with an electrochemical detector was used for this study. For free glycerol determinations, the biodiesel samples were extracted with water and the aqueous layer was removed for analysis. For total glycerol determinations, biodiesel samples were hydrolyzed in 1 M NaOH by refluxing at 95 C for 1 h, cooled, and the aqueous layer removed for
analysis (1).
Results
The method was evaluated by determining the LOD, LOQ, and the linearity of response from seven standards (n = 2, 0.05–10 mg/kg glycerol). The method was sensitive, with an LOD (3× S/N) and LOQ (10× S/N) of 0.5 and 2.4 µg/kg, respectively. The linearity in the specified range produced a correlation coefficient (r2 ) of 0.9998.
To evaluate the method for determining free and total glycerol, we analyzed ten biodiesel samples. The glycerol peak was well resolved from an unknown peak, as shown in the total glycerol determination of a B100 animal-source biodiesel sample (Figure 1). Free glycerol concentrations ranged from 0.02–11.8 mg/kg (n = 2) while total glycerol concentrations ranged from 0.066–188 mg/kg (n = 2). All glycerol concentrations in the biodiesel samples were within the ASTM specifications of <0.02 (200 mg/kg) and 0.24 wt% (2400 mg/kg), respectively. Recoveries of glycerol added at twice the sample concentrations ranged from 92–100%.
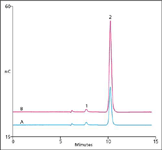
Figure 1: Comparison of total glycerol in an aqueous solution of a base-hydrolyzed B100 animal sample A) without, and B) with 80 mg/kg added glycerol. Sample preparation: base-hydrolysis at 95 °C for 1 h, dilute aqueous layer 1:5, filter 0.2 µm. Column: CarboPac® MA1 guard and analytical; eluent: 100 mM sodium hydroxide; temperature: 30 °C; flow rate: 0.40 mL/min; injection volume: 5 µL; detection: PAD, Au, Waveform A (1). Peaks: 1) Unknown; 2) glycerol: A-0.54 mg/kg, B-1.3 mg/kg.
HPAE-PAD is a sensitive and selective method that does not require the sample derivatization needed for the GC-FID method, and therefore provides a good alternative for determining µg/kg to mg/kg concentrations of glycerol from aqueous extractions of biodiesel samples.
References
(1) Dionex Corporation. Determination of Free and Total Glycerol in Biodiesel Samples by High Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection (HPAE-PAD), Application Note 255, LPN 2571, Sunnyvale, CA (2010).
CarboPac and Chromeleon are registered trademarks of Dionex.
Dionex Corporation
1228 Titan Way, P.O. Box 3603, Sunnyvale, CA 94088
tel. (408) 737-0700, fax (408) 730-9403
Website: www.dionex.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















