Ultra-Fast LC–MS-MS Analysis of 25-OH Vitamin D2 and D3 from Serum
An analytical method for the rapid determination of 25-OH vitamin D2 and 25-OH vitamin D3 in serum, which will speed diagnosis of potential Vitamin D deficiencies, is described.
An analytical method for the rapid determination of 25-OH vitamin D2 and 25-OH vitamin D3 in serum, which will speed diagnosis of potential Vitamin D deficiencies, is described. The method utilizes protein precipitation and ultra-fast LC–MS-MS using a Kinetex 2.6 µm core-shell column.
Experimental
Sample Preparation
1. To a 1.5 mL centrifuge tube add 350 µL of the precipitating reagent containing internal standard.
2. Pipette 100 µL of serum into the centrifuge tube and vortex for 20–30 s.
3. Centrifuge for 15 min at 13,000 rpm.
4. Carefully transfer supernatant into sample vial.
* Alternatively, Strata Impact protein precipitation plates can be used.
Chromatographic Conditions
HPLC System equipped with binary pump, autosampler, and column oven
Column: Kinetex® 2.6 µm C18, 50 × 4.6 mm (Part Number 00B-4462-E0)
Inj. Volume = 50 µL
Flow rate: 1.0 mL/min
Mobile Phase: A = 0.05 % formic acid in water B = 5 mM ammonium acetate + 0.1% formic acid in methanol
Gradient: 8% B to 100% B from 5 to 205 s; hold 85 s; re-equilibrate for 70 s at 8% B
Temperature: 35 °C
Detector: APCI in positive ion mode
Results and Discussion
Analysis of 25-OH D2 and 25-OH D3 from serum necessitates the use of sample preparation to remove potential matrix constituents, which will interfere with accurate and precise determination of 25-OH D in serum and reduce HPLC column lifetime. Protein precipitation is the easiest means of disrupting the serum protein association with analytes and found to be sufficient for sample preparation of serum samples prior to LC–MS-MS determination of 25-OH D2 and 25-OH D3.
LC–MS-MS was performed in multiple reaction monitoring (MRM) mode. The use of MRM is important since the 25-OH D2 and 25-OH D3 are not fully separated chromatographically, however, the unique parent/daughter ion combination for each analyte allows for specificity and accurate determination of the concentration for each analyte in the sample.
25-OH D2 and 25-OH D3 elute in less than 0.5 min on the Kinetex core-shell C18 column with sufficient resolution to further minimize the potential for interference and ion suppression from weakly retained impurities (Figure 1). Total chromatographic cycle time is 6 min, including column cleaning and reequilibration. The fast analysis time using the Kinetex core-shell column is significant for laboratories analyzing a large number of samples in a high throughput sample environment. The presence of an endogenous compound (D3 Cholesterol) in patient samples was resolved from 25-OH D3 on the Kinetex column improving accuracy and precision.
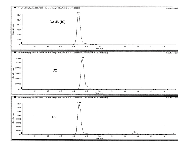
Figure 1: Ultra-fast, high-sensitivity LCâMS-MS analysis of 25-OH D2 and D3 in serum. Individual MRM transitions monitored:25-OH D2, 383.2/257.2; 25-OH D3, 395.3/209.3; 25-OH D3-d6,389.3/263.3.
Acknowledgments
Sample preparation and LC–MS-MS conditions are courtesy of Kaiser Permanente Southern California Regional Laboratory.
Phenomenex, Inc.,
411 Madrid Avenue, Torrance, CA 90501
tel. (310) 212-0555, fax (310) 328-7768
Website: www.phenomenex.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















