Summarization of Screening Hits on the Whelk-O 1, RegisPack, and RegisCell Chiral Stationary Phases (CSPs)
The determination of enantiomeric purity is crucial in new drug development.
Ted Szczerba, Regis Technologies, Inc.
The determination of enantiomeric purity is crucial in new drug development. The number of diverse chiral compounds is increasing and sensitive chiral methods are often needed quickly. With many new CSPs on the market, it is challenging to select the most important ones for the initial screening stages and to expedite method development. The focus of this study is to evaluate high selectivity Regis CSPs and to suggest the best screening method with a limited number of high success rate chiral columns.
Five-hundred and nineteen test compounds were screened on a number of different CSPs. These are client-submitted samples for Regis' free chiral screening service. The test library consists of active pharmaceutical ingredients (APIs), potential drug candidates, proprietary research compounds, or simple commercially available compounds.
While we decided to summarize results for all the samples screened, a number of these compounds are unique and there is no guarantee that they are truly chiral. It has been proven on numerous occasions that some of the samples submitted are achiral and cannot be separated on chiral columns.
The focus was on three of the most versatile columns on the market. They consist of the Whelk-O®1, RegisPack®, and RegisCell® CSPs. These three phases show a high success rate and broad versatility. In addition, these three CSPs are complementary in selectivity, as demonstrated by the number of unique hits for each CSP. All the columns were standard dimensions, 25 cm × 4.6 mm i.d. packed with a 5 micron particle size. Each compound was screened individually and a number of different mobile phases were applied during the screening process to achieve optimal separation.
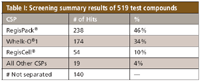
Table 1
# of hits on multiple CSPs
Whelk-O®1 and RegisPack® – 63
Whelk-O®1 and RegisCell® – 9
RegisPack® and RegisCell® – 12
Whelk-O®1, RegisPack®, and RegisCell® – 13
# of unique hits
Whelk-O®1 – 89
RegisPack® – 150
RegisCell® – 20
Conclusion
Out of a total of 519 compounds screened, 379 compounds were separated on numerous CSPs. Out of the 379 compounds separated, 360 compounds were separated on either the Whelk-O®1, RegisPack®, or RegisCell® CSPs for a hit ratio of 95%. Some compounds were separated on more than one column (See Figure 1). These three CSPs account for a 70% success rate out of the total of 519 compounds screened.
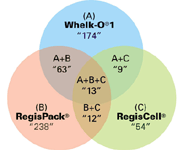
Figure 1: Graphic illustration of the screening success rate and the summary of results.
With the Whelk-O®1, RegisPack®, and RegisCell® columns installed on a screening station, the chromatographer can expect greater than a 70% success rate in the screening process.
NOTE
If we were guaranteed that all of the compounds were truly racemic, the success rate would most likely be higher.
Regis Technologies, Inc.
8210 Austin Ave., Morton Grove, IL 60053
tel. (847) 583-7661, fax (847) 967-1214
Email: teds@registech.com, Website: www.registech.com/chiral

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















