Recent Advances in Silica-Based Monolithic HPLC Columns
In this article, silica-based monolithic columns are compared and contrasted to packed microparticulate columns. Some of the challenges developing commercial silica rods and encapsulated monolith columns are described, including the development of a 2-mm i.d. column. A study of wall effects in these monolith columns was performed. Future trends and challenges in improving the performance of silica-based monolith columns are described.
In this article, silica-based monolithic columns are compared and contrasted to packed microparticulate columns. Some of the challenges developing commercial silica rods and encapsulated monolith columns are described, including the development of a 2-mm i.d. column. A study of wall effects in these monolith columns was performed. Future trends and challenges in improving the performance of silica-based monolith columns are described.

In contrast to conventional particle-packed high performance liquid chromatography (HPLC) columns, monolithic columns are made of a continuous piece of either porous silica or organic polymer. Their preparation and chromatographic characterization has been reviewed recently (1–6). Both types of the new generation of monolithic columns are now available commercially and are under intensive investigation. This article will focus only on silica-based monoliths and describe from the author´s perspective "where we are" from a scientific and commercial point of view. Organic polymeric monoliths are reviewed by Svec and Krenkova in a separate paper in the same issue of this journal (7).
Silica-based monolithic columns are manufactured utilizing a sol-gel process (8) leading to rod columns, which possess a defined bimodal pore structure with macro and meso pores in the micro- and nanometer range (ca. 2 μm and 13 nm, respectively). The macro pores are equivalent to the interstitial volume of particle-packed columns, which determines the permeability of a column. The mesopores are located either in the silica skeleton of monoliths or on the silica particle surface. These porous structures give high surface areas for monolithic and microparticulate silicas, which are necessary for efficient separations in adsorption chromatography.
Silica-based monolithic columns have been available commercially since 2000, initially with analytical columns of 4.6 mm internal diameter (i.d.), followed with 3- and 10-mm i.d. products along with a preparative 25-mm i.d. version. Figure 1 provides a pictorial representation of commercially available silica rod columns. Recently, a 2-mm i.d. silica monolith column (Chromolith FastGradient, Merck KGaA /EMD Chemicals, Inc., Gibbstown, New Jersey) has been introduced at the PittCon 2008 in New Orleans. Generally, monolithic silica columns with internal diameters in the millimeter range cannot be manufactured in-situ — directly inside of a column tube — because of shrinkage during the sol-gel process. The silica rods must be synthesized outside of the column and then be encapsulated in a solvent- and pressure-resistant polymer tube to be used for HPLC. In contrast, monolithic silica capillaries with small diameters (for example, 100-μm i.d.) can be prepared in-situ and these smaller diameter monolith columns also are available commercially. However, this article will focus only on silica-based monoliths with internal diameters ≥ 2 mm.

Figure 1
A unique feature and big advantage of silica-based monolithic HPLC columns over packed microparticulate columns is the ability to control the macro and meso pore sizes independently as well as the silica skeleton size. This control is achieved through the sol-gel preparation process. As a result, this process permits the design of HPLC columns that show simultaneous high separation efficiencies and high permeabilities, not entirely possible with most packed-particle columns. In the latter case, the particle size determines the separation efficiency and permeability but in a reciprocal manner, that is, large particles lead to high permeabilities and low efficiencies, whereas small particles lead to the opposite relation. Superficially porous particle columns can provide high efficiency with lower permeability (9). The high separation efficiency and simultaneous high permeability of silica-based monolithic columns allows their fast operation with conventional low-pressure HPLC systems and using higher flow rates, most commonly 1–5 mL/min. This intrinsic advantage of silica-based monolithic columns has been demonstrated by numerous applications (10), including high-throughput analysis, separation of environmentally relevant substances, food additives, ions and enantiomers as well as complex biological samples, for example, tryptic digests.
Since becoming available, silica-based monoliths have been characterized and compared to packed microparticulate columns from both a theoretical and practical point of view (11–19). The results of these investigations can be summarized as follows: the external porosity obtained either by the macro–throughpores of monoliths or by the interstitial volume of particle-packed columns is almost double in the case of monoliths providing HPLC columns with high permeabilities (that is, low column back pressures); column efficiency of standard silica-based monoliths is comparable to 3.5-μm packed microparticulate columns; column efficiency is influenced by the domain size ddom (sum of throughpore diameter and silica skeleton diameter), which is more or less equivalent to the particle diameter dp; the mesopore size and corresponding surface area is similar for both types of columns; and the internal porosity (due to the mesopores) is lower in monoliths, which is a result of their high porosity and corresponding low density.
A number of authors (20–23) have attempted to develop theoretical models to calculate and improve the porous silica structure of monoliths with respect to column efficiency. Vervoort and colleagues (22) showed that the domain size is a good reference for the HETP (height equivalent to a theoretical plate) and that a value of the reduced HETP (h = H/ddom) as low as 0.8 should be obtained for a highly homogeneous rod. In contrast to the theoretical considerations, the experimental data shows much higher values. It is anticipated that the eddy diffusion (A-term of van Deemter equation) is markedly larger with monolithic than with particle-packed columns. Apparently, this increase in eddy diffusion has something to do with the heterogeneity of the monolithic porous silica structure itself and possibly also with the radial heterogeneity of the monolithic columns (10). Higher efficiencies probably can be achieved in the near future if the understanding of silica-based monolith columns is improved by more intensive studies of these issues.
Challenges in the Development of Silica-Based Monolithic HPLC Columns
The quality of silica-based monolithic columns is influenced mainly by the porous silica network of the monolith itself, the type of surface modification, and the quality of encapsulation with a polymer. The latter was one of the big hurdles to take during the development of silica-based monolithic columns. The challenge was to tightly melt a polymer on the outer surface of the silica monoliths with no destruction of the silica structure and no voids at the interface thereby eliminating "wall effects." Figure 2 shows an SEM picture of the interface of a silica-based monolithic column with perfect cladding using PEEK (polyetheretherketone) polymer with no voids along the wall. An optimum cladding is the prerequisite for good column performance. It affects the separation efficiency and peak symmetry to a large extent. The smaller the diameter of a column, the more pronounced interface/ wall regions will affect the overall performance of the column negatively.

Figure 2
In 1976, Knox and colleagues published a paper (24) studying the influence of the wall region of packed microparticulate columns on its overall performance. To test for the presence of wall effects of micro-particulate columns, a dedicated HPLC injection system was constructed, which permitted the isolated injection of a sample in the center and in the wall region of a packed microparticulate column. It was concluded from the experiment that the efficiency of a packed microparticulate column is three times less in the wall region as compared to the center, most likely due to a lower packing density.

Figure 3
Performing similar experiments with an optimum cladded silica monolith column, the chromatographic data showed that efficiency was of the same magnitude in the center and along the interface–wall region of the column. Figure 3 shows the overlap of three chromatograms for three repeated injections of a sample mixture with 2-nitroanisole as the third eluted peak in the center and wall region of an unmodified silica monolith. The chromatographic performance data observed in the center and in the wall region are very similar, as can be seen in Table I. The deviations are due to the manual injections on top of the column with a syringe after replacement of the end fitting.
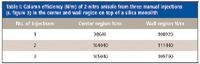
Table I: Column efficiency (N/m) of 2-nitro anisole from three manual injections (s. figure 3) in the center and wall region on top of a silica monolith
Development and Application of Narrow-Bore 2-mm i.d. Chromolith Columns
After the market introduction of 4.6-mm i.d. monolithic silica columns (Chromolith, Merck KGaA), it was a challenge to develop an equivalent 2-mm i.d. column because of the technical reasons described previously. By overcoming these barriers, it is now possible to manufacture silica-based monoliths of small diameters (2-mm i.d.) analogous to the 3.0- and 4.6-mm i.d. columns in a reproducible manner and with expected chromatographic performance. These 2-mm i.d. monolithic columns especially are welcomed by scientists performing LC–mass spectrometry (MS) who prefer splitless operational modes. With the 2-mm i.d. columns, the flow rates are typically under 1 mL/min in line with the requirements of electrospray ionization and with acceptable separation times. In addition, the smaller internal diameter columns save solvent for conventional LC–UV users due to smaller column volumes. The biggest advantage of the monolithic 2-mm i.d. columns is the lower pressure drop compared with packed microparticulate columns of the same performance level. Thus, longer columns can be used if high plate counts are required. Another big advantage of the 2-mm i.d. silica monolith over small packed microparticulate columns (1.5–2.5 μm) is the possibility of their use in conventional low-pressure HPLC systems instead of ultrahigh-pressure systems. Due to smaller peak volumes, however, the HPLC system must be adapted in its operation with these smaller diameter columns, such as installation of a suitable smaller volume detector cell and perhaps smaller diameter connecting tubings, adaption of detection response times, and optimized data system settings for the narrow, quickly eluted peaks. Such an optimized conventional HPLC–UV system offers a much higher sensitivity, by a factor of 5.3 using the 2-mm i.d. column instead of the commonly used 4.6-mm i.d. ones. Increased sensitivity and speed typically are needed in the area of pharmaceutical analysis. Figure 4 shows an example of the chromatography of steroid substances that are relevant in the field of doping. Currently, doping plays an important role in sporting activities, for example, in the Olympic Games, which will take place in Beijing, China this year. Fast HPLC methods are needed to detect such "forbidden" substances in small amounts now possible with the 2-mm i.d. columns in the seconds to minutes timeframe.

Figure 4
Conclusions and Future Trends
The use of silica-based monolithic columns will continue to increase because of their inherent good properties. Silica-based C18 monolithic columns are listed in the U.S. Pharmacopeia as an "L1" column. Indeed, a comparative column characterization study, published on the website of the U.S. Pharmacopeia (25), reveals that silica monoliths are equivalent to many different packed microparticulate columns whose equivalency has been confirmed by others (26). This fact has motivated many workers in the pharmaceutical industry strongly (27–32), and many have used silica monoliths for method development of R&D and QC applications. Further available data on batch-to-batch reproducibility of silica monoliths has been described (33–35).
From a scientific point of view, it is expected that the column performance of silica-based monoliths will be further improved. Optimization of the sol-gel process with respect to the structure directing components (silane precursor and pore-forming template) will lead to more efficient monoliths. Tanaka and colleagues (36–39) have developed numerous new materials by a systematic variation of the macro-throughpore size in combination with the silica skeleton size. The resulting monoliths provide smaller domain sizes and higher chromatographic performance. Furthermore, the homogeneity of the porous silica structure as well as the radial homogeneity of monolithic columns must be improved to achieve even higher column performance. This task is not a trivial one but can be solved by the manufacturers and academic researchers working together.
Finally, a good HPLC column is characterized by a high column performance in addition to a sufficient selectivity, which is influenced by the surface modification process. Numerous research and development activities are focusing on monolithic HPLC columns with new surface modifications and resulting new selectivities. Among these activities is the development of hydrophilic interaction chromatography (HILIC)-type surface modifications in several research groups (40–42). Silica-based monoliths with HILIC-type surface modifications are suited for the fast separation of highly polar substances especially, including biologically active compounds such as pharmaceutical drugs, neurotransmitters, nucleosides, nucleotides, amino acids, peptides, proteins, oligosaccharides, carbohydrates, etc. Because the need for the analysis of these types of compounds in the life science area is increasing dramatically, HILIC columns will be a valuable separation tool in the future.
Acknowledgment
The author would like to thank the Chromolith-Team at Merck KGaA (Darmstadt) for their continuous engagement in the development of new monolithic materials. Special thanks are to H.D. Pohl and K. Kühne for intensive discussions and support on the manual injections according to Knox and colleagues (22). Special thanks also to S. Olbrich for providing Figure 4.
References
(1) G. Guiochon, J. Chromatogr., A 1168, 101–168 (2007).
(2) A.-M. Siouffi, J. Chromatogr., A 1000, 801–818 (2003).
(3) H. Kobayashi, T. Ikegami, H. Kimura, T. Hara, D. Tokuda, and N. Tanaka, Anal. Sci. 22(4), 491–501 (2006).
(4) F. Svec and C. Huber, Anal. Chem. 78(7), 2100–2108 (2006).
(5) F. Svec, J. Sep. Sci. 27(17–18), 1419–1430 (2004).
(6) F. Svec and J.M.J. Frechet, in Monolithic Materials: Preparation, Properties and Applications, F. Svec, T.B. Tennikova, and Z. Deyl, Eds. (Elsevier, Amsterdam, 2003).
(7) F. Svec and Krenkova, LCGC 26(S4) (2008).
(8) K. Nakanishi, J. Por. Mat. 4, 67–112 (1997).
(9) J.J. De Stefano, T.J. Langlois, and J.J. Kirkland, J. Chrom. Sci. 46(3), 254–260 (2008).
(10) K. Cabrera, J. Sep. Sci. 27, 843–852 (2004).
(11) H. Minakuchi, K. Nakanishi, N. Soga, N. Ishizuka, and N. Tanaka, J. Chromatogr., A 762(1–2), 135–146 (1997).
(12) H. Minakuchi, K. Nakanishi, N. Soga, N. Ishizuka, and N. Tanaka, J. Chromatogr., A 797(1–2), 121–131 (1998).
(13) H. Minakuchi, K. Nakanishi, N. Soga, N. Ishizuka, and N. Tanaka, J. Chromatogr., A 797(1–2), 133–137 (1998).
(14) F.C. Leinweber, D. Lubda, K. Cabrera, and U. Tallarek, Anal. Chem. 74(11), 2470–2477 (2002).
(15) F.C. Leinweber and U. Tallarek, J. Chromatogr., A 1006, 207–228 (2003).
(16) K. Miyabe and G. Guiochon, J. Sep. Sci. 27(10–11) 853–873 (2004).
(17) S. Eeltink, W.M.C. Decrop, G.P. Rozing, P.J. Schoenmakers, and W.T. Kok, J. Sep. Sci. 27(17–18), 1431–1440 (2004).
(18) S. Eeltink, P. Gzil, W.T. Kok, P.J. Schoenmakers, and G. Desmet, J. Chromatogr., A 1130(1), 108–114 (2006).
(19) S. Eeltink, G. Desmet, G. Vivo-Truyols, G.P. Rozing, P.J. Schoenmakers, and W.T. Kok, J. Chromatogr., A 1104(1–2), 256–262 (2006).
(20) K. Miyabe, A. Cavazzini, F. Gritti, M. Kele, and G. Guiochon, Anal. Chem. 75, 6975–6986 (2003).
(21) K. Miyabe and G. Guiochon, J. Phys. Chem. B, 106, 8898 (2002).
(22) N. Vervoort, P. Gzill, G.V. Baron, and G. Desmet, J. Chromatogr., A 1030, 177 (2004).
(23) J. Billen, P. Gzil, G.V. Baron, and G. Desmet, J. Chromatogr., A 1077, 28 (2005).
(24) J. H. Knox, G.R. Laird, P.A. Raven, J. Chromatogr. 122, 129–145 (1976).
(25) www.usp.org/USPNF/columnsDB.html
(26) K. Koczian, E. Haghedooren, S. Dragovic, B. Noszal, J. Hoogmartens, and E. Adams, J. Pharm. Biomed. Anal. 44(3), 634–639 (2007).
(27) R. Muniz-Valencia, R. Gonzalo-Lumbreras, A. Santos-Montes, and R. Izquierdo-Hornillos, J. Sep. Sci. 30(17), 2950–2957 (2007).
(28) Y.H. Ardakani and M.-R. Rouini, J. Pharm. Biomed. Anal. 44(5), 1168–1173 (2007).
(29) G. Savic, M. Zecevic, B. Jocic, and L. Zivanovic, Chromatographia, 66(1/2), 29–35 (2007).
(30) S. El Deeb, L. Preu, H. Wätzig, J. Pharm. Biomed. Anal. 44(1), 85–95 (2007).
(31) P.D. Tzanavaras and D.G. Themelis, J. Pharm. Biomed. Anal. 43(4), 1483–1487 (2007).
(32) N. Wu, J. Dempsey, P.M. Yehl, A. Dovletoglu, D. Ellison, and J. Wyvratt, Anal. Chim. Acta, 523(2), 149–156 (2004).
(33) M. Kele, G. Guiochon, J. Chromatogr. A, 960(1–2), 19–49 (2002).
(34) H. Engelhardt, A. Goetzinger, Chromatographia, 60(1), 207–211 (2004).
(35) S. El Deeb, U. Schepers, H. Waetzig, Pharmazie 61(9), 751–756 (2006).
(36) N. Ishizuka, H. Kobayashi, H. Minakuchi, K. Nakanishi, K. Hirao, K. Hosoya, T. Ikegami, and N. Tanaka, J. Chromatogr. A, 960(1–2), 85–96 (2002).
(37) M. Motokawa, H. Kobayashi, N. Ishizuka, H. Minakuchi, K. Nakanishi, H. Jinnai, K. Hosoya, T. Ikegami, and N. Tanaka, J. Chromatogr., A 961(1), 53–63 (2002).
(38) N. Tanaka, H. Kobayashi, N. Ishizuka, H. Minakuchi, K. Nakanishi, K. Hosoya, and T. Ikegami, J. Chromatogr., A 965(1–2), 35–49 (2002).
(39) T. Hara, H. Kobayashi, T. Ikegami, K. Nakanishi, and N. Tanaka, Anal. Chem. 78(22), 7632–7642 (2006).
(40) P. Hemström, K. Irgum, J. Sep. Sci. 29, 1784–1821 (2006).
(41) T. Ikegami, K. Tomomatsu, H. Takubo, K. Horie, and N. Tanaka, J. Chromatogr., A 1184(1–2) 474–503 (2008).
(42) K. Oiré, T. Ikegami, K. Hosoya, N. Saad, O. Fiehn, and N. Tanaka, J. Chromatogr., A 1164(1–2) 198–205 (2007).

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.
A Guide to (U)HPLC Column Selection for Protein Analysis
April 16th 2025Analytical scientists are faced with the task of finding the right column from an almost unmanageable range of products. This paper focuses on columns that enable protein analysis under native conditions through size exclusion, hydrophobic interaction, and ion exchange chromatography. It will highlight the different column characteristics—pore size, particle size, base matrices, column dimensions, ligands—and which questions will help decide which columns to use.














