High-Temperature Liquid Chromatography
The use of high temperature is playing an increasingly important role in high performance liquid chromatography (HPLC) method development and optimization. Major advantages of high-temperature LC (HTLC) include shortened separation time, increased efficiency, and reduction in the use of organic solvent, but the accompanying decrease in mobile phase viscosity provides a lowering of column back pressure, allowing even faster separations, use of longer columns, and use of smaller particles. Here the author summarizes some of the latest findings in HTLC and addresses issues raised when columns and analytes are heated beyond the "normal" operating conditions.
The use of high temperature is playing an increasingly important role in high performance liquid chromatography (HPLC) method development and optimization. Major advantages of high-temperature LC (HTLC) include shortened separation time, increased efficiency, and reduction in the use of organic solvent, but the accompanying decrease in mobile phase viscosity provides a lowering of column back pressure, allowing even faster separations, use of longer columns, and use of smaller particles. Here the author summarizes some of the latest findings in HTLC and addresses issues raised when columns and analytes are heated beyond the "normal" operating conditions.
In addition to the traditional liquid chromatography parameters such as the composition, pH, and ionic strength of the mobile phase; the type, particle and pore size of the stationary phase; and column dimensions, temperature has gained greater attention as another important variable in high performance liquid chromatography (HPLC) method development during the last decade.
High-temperature liquid chromatography (HTLC) offers several advantages when compared with the traditional room-temperature HPLC. The benefits of HTLC include greatly shortened separation and analysis time; improved column efficiency; minimization or elimination of the use of organic solvents involved in the mobile phase; compatibility with longer columns packed with even smaller particles; and the ability to perform temperature-programmed elution. Selectivity changes can occur but these can have a positive or negative effect on resolution because relative retention of analytes can be affected by changes in temperature. The only limitation for HTLC is the stability of both stationary phases and analytes under elevated temperature conditions.

The following issues are addressed in this paper: fundamental aspects for temperature optimization in HPLC method development; temperature effects on separation speed, selectivity, resolution, and efficiency; the stability of stationary phases and analytes under HTLC conditions; temperature control in HTLC systems; application of high temperature in multicolumn and multidimensional HPLC techniques; chromatographic separation using subcritical water as the sole eluent; HPLC separations at subambient temperatures; and the challenges and limitations for the HTLC technique.
Temperature — Another Important Variable in HPLC Method Development
Although HPLC has become an important and popular separation and analysis technique for many decades, it was not until the 1990s that the separation temperature started gaining increased attention as a parameter in HPLC optimization (1–5). The dielectric constant and surface tension of the mobile phase play important roles in LC retention, and they both are highly temperature-dependent. In reversed-phase LC, the dielectric constant and surface tension of the mobile phase mixtures (for example, water–methanol or water–acetonitrile) significantly decrease with increasing temperature. The lowered dielectric constant and surface tension of the eluent allow dramatic decrease in solute retention at elevated temperatures as indicated in the van't Hoff plot, where the natural logarithm of the retention factor is inversely proportional to the absolute temperature, ln k α 1/T. While the dielectric constant, surface tension, and viscosity of the reversed-phase LC eluent can be tuned over a wide range of temperature, the reported temperature conditions for reversed-phase LC separations vary from subambient to ~350 °C. However, the most frequently used temperature in HTLC normally ranges from ~50 °C to ~150 °C.
Beneficial Temperature Effects on HTLC Separations
As described previously by the van't Hoff plot, solute retention is shortened dramatically with increasing temperature. This leads to fast separation and analysis offered by the HTLC technique. In addition, the reduced viscosity of the mobile phase at elevated temperatures results in much lower back pressure in an HTLC system. An ongoing HTLC project in the author's research laboratory shows that the back pressure of an HTLC system decreases over threefold by simply raising the temperature from 25 °C to 90 °C when a mixture of 40% methanol and 60% water is used. It also was reported that the back pressure was reduced by fivefold over a temperature range of 25–180 °C in subcritical water chromatography, where pure water was used as the mobile phase (6). The combined effects of high temperature and low pressure make it possible for HTLC to use very high flow rates to achieve fast separations, even within seconds as shown in Figure 1 (7), a truly ultrafast LC separation technique. The fast HTLC separation can be achieved using ordinary HPLC pumps with a maximum pressure of 400 bar because of the much lowered back pressure of the eluent at high temperatures. For example, 400 bar can allow only 2–3 mL/min flow rates for a 25 cm × 4.6 mm column packed with 5-μm particles at ambient temperature, but the same HPLC system allows the use of much higher flow rates at 150–200 °C.

Figure 1
As we all know, the column efficiency is enhanced when smaller particles are used. Unfortunately, the back pressure of the HPLC system is inversely proportional to the square of the particle diameter and, thus, dramatically increases with decreasing particle size. Therefore, at ambient temperature, only short columns packed with 3-μm (or smaller) packings can be used in HPLC systems equipped with ordinary pumps with a maximum pressure of 400 bar. However, using the same conventional HPLC pump, longer columns packed with smaller particles can be tolerated at higher temperatures because of the significantly reduced back pressure that results from the much-lowered viscosity of the eluent at elevated temperatures. This leads to enhanced separation efficiency, which results from the smaller packing particles and the increased length of the column.
Nonlinear van't Hoff plots frequently are reported in the literature, demonstrating that the solutes not separated at low temperatures can be separated at a higher temperature. Thus, selectivity and resolution can be improved in HTLC if the separation temperature is optimized properly. Figure 2 shows chromatograms of steroids on a poly(N-isopropylacrylamide) terminally modified column at 10 °C and 50 °C (8). It is clear that separation of steroids was not achieved at 10 °C, but the analytes were well separated at 50 °C. Both selectivity and resolution were improved greatly at higher temperature in this case.
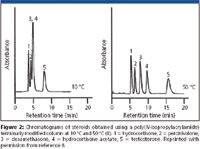
Figure 2
Because of the enhanced mass transfer at higher temperatures, the peak broadening C term in the van Deemter equation is reduced greatly, leading to much narrower peaks. However, the retention time also decreases dramatically with rising temperature, as discussed earlier. Because the plate number is determined by the ratio of retention and peak width, the temperature effect on column efficiency is a complicated one (9). But, it is clear that the column efficiency can be optimized by tuning the temperature in addition to the traditional optimization of the linear velocity of the eluent (9). The better mass transfer at elevated temperatures also allows elution with programmed temperature to further enhance the quality of HTLC separation.
The consumption of organic solvents in the mobile phase can be reduced, saving costs in purchasing and disposal of the organic solvents used in the mobile phase. In the case of subcritical water chromatography, in which water is used as the sole eluent, organic solvents are eliminated completely in the mobile phase. Therefore, LC separation using high-temperature water is a "green" separation technique.
Stationary Phases Tested for HTLC Separations
The stationary phases investigated in HTLC were reviewed recently (10–12). In general, some silica-bonded packings show good long-term stability at temperatures around 100 °C, although many of them cannot tolerate such temperatures. Zirconia- and other metal oxide-based columns normally can be used at temperatures ranging from 100 °C to 150 °C for several thousand column volumes without significant degradation of the stationary phases. Among all existing LC packing materials, polymer stationary phases are the most stable ones, and can be used reliably at high temperatures, typically at 150 °C or higher. However, the efficiency of polymer columns is poorer that that of silica- and zirconia-based columns (10–12).
Other stationary phases tested in HTLC include carbon, monolithic, and temperature-responsive packings. Specially designed HPLC chips also were used for LC separations at various temperatures (13). In addition to the standard and microbore columns, packed capillary and open tubular columns also have been used in HTLC, and they are more appropriate for separations with programmed temperature. Please see references 10–12 for details regarding the stationary phases used in HTLC.
Analyte Stability Under HTLC Conditions
While the harsh temperature conditions involved in HTLC do cause problems for many packing materials, especially the silica-based stationary phases, most analytes are stable on the time scale of the HTLC run. As discussed in previous sections, the combined effects of high temperature and fast flow rate dramatically reduce the separation and analysis time. Therefore, HTLC separation shortens the analyte exposure time to high temperature, and in turn minimizes analyte degradation. Even some pharmaceuticals can withstand high temperatures on the time scale of fast LC separation (14). As the solute retention time on a hot column decreases, the extent of on-column reaction decreases for those analytes that do react (14). Thus, analyte degradation is not a major concern in HTLC.
Thermal Mismatch, Heating, and Cooling in HTLC
Because the analytical column is heated in HTLC at a temperature higher than that of the mobile phase reservoir, the low temperature of the incoming mobile phase does not match the high temperature of the separation column if the eluent is not preheated efficiently. This thermal mismatch causes poor reproducibility and other separation problems.
To minimize thermal mismatch, several approaches have been reported. For example, a preheating coil is placed inside an oven or a heating unit between the injector and the column. While this approach can reduce or even eliminate thermal mismatch, it causes additional peak broadening due to the added void volume of the preheating coil.
A specially designed heating system for a temperature-programmed HTLC system was developed based upon experimental measurements of eluent temperature inside a stainless steel capillary using very thin thermocouples (15). As shown in Figure 3, a preheating unit is employed to heat the mobile phase efficiently before it enters the column. Again, although the thermal mismatch is eliminated, the extra tubing located inside the preheating unit still contributes to minor additional peak broadening. Because the preheating tubing used in Figure 3 can be shorter than that used in the first approach, the second approach should yield more efficient HTLC separation.
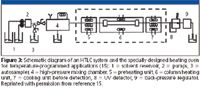
Figure 3
To overcome the additional peak-broadening problem resulted by the first two approaches as described previously, the preheating coil can be placed inside an oven, but between the pump and the injector that is located outside the oven. Because the analytes are injected into the column without passing through the preheating coil, additional peak broadening is eliminated. However, thermal mismatch may not be minimized effectively in this approach because the injector outside the oven acts as a cooling device that lowers the temperature of the preheated eluent.
A better experimental set-up that can eliminate both thermal mismatch and additional band broadening is demonstrated in Figure 4 (16). While the use of additional pumps connecting to the preheating coil eliminates thermal mismatch, the band-broadening problem caused by the preheating coil also is eliminated in this approach. A separate pump (the top pump in Figure 4) enables the injected analytes to bypass the preheating coil and be delivered directly to the column (16). The drawback of this approach is the additional pump or pumps required to deliver the mobile phase through different channels. Some HPLC manufacturers have developed commercial HTLC–HPLC systems that allow preheating of the mobile phase. For example, the Agilent 1200 system has a built-in preheater (Model G1316, Agilent Technologies, Santa Clara, California) that can preheat the mobile phase up to 100 °C to eliminate thermal mismatch.
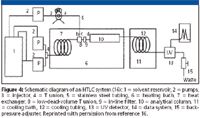
Figure 4
Although several detection techniques have been used in HTLC, UV detection is the widely employed one. Because most UV flow cells are not designed for high-temperature applications, the eluent must be cooled down before reaching the UV detector, as shown in Figures 3 and 4. Just like the preheating coil, the cooling coil also leads to additional band broadening; however, some modern postcolumn cooling coils have only a few microliters of dead volume. However, this problem is eliminated in subcritical water chromatography, in which flame ionization detection (FID) is employed and the cooling of the eluent between the column and the FID is not required (12).
Applications of High Temperature in Tandem-Column and Two-Dimensional HPLC Techniques
Selectivity is an important issue in LC and it depends upon the property of the stationary phase and the mobile phase, pH, and temperature. While it is common to adjust mobile phase composition, pH, and temperature continuously to achieve more selective separation, continuously adjusting the stationary phase is more difficult and less common. However, a thermally tuned tandem column approach allows continuous adjustment of the stationary phase, the mobile phase, pH, and temperature (17). In this approach, two columns with distinctly different chromatographic selectivity were serially connected, and independent temperature control was used for the columns (17). Figure 5 shows the chromatograms for separation of barbiturates using a C18 column at 30 °C (Figure 5a), a carbon-coated zirconia column at 30 °C (Figure 5b), and a thermally tuned tandem column set with a C18 column at 80 °C and a carbon-coated zirconia column at 40 °C (Figure 5c). The better selectivity offered by the thermally tuned tandem column approach is seen clearly. The thermally tuned tandem column system enables convenient and fast method development. Only minor modifications to commercial HPLC instruments are necessary to perform the thermally tuned tandem column separation.

Figure 5
According to the statistical model of overlap theory, the resolving power of one-dimensional HPLC is insufficient, especially for bioanalytical applications. Thus, two-dimensional HPLC is desired for peptide and protein separations. However, two-dimensional HPLC separations require very long analysis time per run. Recently, high temperature has been applied to two-dimensional HPLC separations (18). The application of high temperature greatly reduced the run time, thus, providing fast and comprehensive bioanalysis.
Subcritical Water Chromatography
Subcritical water refers to the water that is heated and pressurized at conditions below its critical point of 374 °C and 218 atm. Other terminologies used for water under subcritical conditions include superheated water, pressurized hot water, and high-temperature water.
The mobile phase organic solvents required for reversed-phase LC separations are expensive and toxic, and proper disposal is required. Compared with these organic solvents, water is environmentally benign, but it is too polar to serve as the sole eluent for reversed-phase separations at ambient temperature. Fortunately, the polarity of water is decreased dramatically with increasing temperature. As shown in Figure 6, simply increasing the water temperature from 25 °C to 250 °C causes similar changes in solvent polarity, surface tension, and viscosity as those achieved by conventional mixing of methanol or acetonitrile with ambient water as done in traditional reversed-phase LC (19). Therefore, subcritical water can mimic the traditional organic solvent–water elution to achieve reversed-phase separation (12).

Figure 6
A unique characteristic for subcritical water chromatography is its compatibility with both gas- and liquid-phase detectors. A well-known limitation for traditional reversed-phase LC is the lack of a sensitive and universal detector, such as the flame ionization detector used in gas chromatography. Unfortunately, FID cannot be used in traditional reversed-phase LC because the organic solvents presented in the eluent cause significant background signal. However, FID has no response to water and, thus, it can be used as a gas-phase detector in LC with water as the sole eluent (20–25). In some of the subcritical water chromatography–FID applications, FID was modified to achieve reliable detection (22). However, nonmodified FID also was tested (20). The problem with this direct coupling technique is the limited volume flow rate of the subcritical water eluent. The FID signal was found to be unstable at water flow rates greater than 0.2 mL/min (20). To solve this problem, a split subcritical water chromatography–FID system was developed (21). The high-temperature water eluent was split before reaching the detector. This split system allows much higher volume flow rates of water, but a stable FID signal still can be maintained. While this split approach works fine for analytes with adequate concentrations, it is not appropriate for analytes with low concentrations because only a small fraction of solute is detected by FID. Thus, another approach to ensure a stable FID signal is the use of microbore or capillary columns that require much lower eluent flow rate to achieve optimized separation (22,23,25).
Similar to other HTLC systems, UV is the most popular detector used in subcritical water chromatography. This is because an subcritical water chromatography–UV system can easily be constructed by modifying a regular HPLC–UV system. A back pressure regulator or a restrictor is attached to the outlet of the UV detector to keep the water inside the separation column in the liquid phase at elevated temperatures. As discussed previously, the hot water eluent normally is cooled down in a cooling unit before it reaches the UV flow cell. This can cause deposition of moderately polar and nonpolar solutes in the transfer tubing as well as additional band broadening. In addition to the economical UV detector, more expensive spectroscopic detection techniques such as nuclear magnetic resonance (NMR) spectroscopy, mass spectrometry, and IR also were tested in subcritical water chromatography (26–28). Please see reference 12 to learn more about subcritical water chromatography.
HPLC Separations at Subambient Temperatures
So far, we have concentrated on HPLC separations at elevated temperatures. However, sometimes the opposite temperature, sub-ambient temperature, is required to achieve better HPLC separation. Therefore, temperature optimization in HPLC should include both sub- and above-ambient conditions. The low-temperature HPLC is more appropriate for separation of pharmaceuticals and biomolecules. There are commercial HPLC systems equipped with precise temperature control to the subambient temperature region.
Challenges and Limitations
The major challenge in HTLC method development is the stability of the column packings under high-temperature conditions. The stationary phases must be stable to ensure the quality of HTLC separation and analysis. Ideal HTLC stationary phases should offer long-term stability at elevated temperatures, but still yield high-column efficiency.
Efficient heating and precise temperature control are critical in HTLC. Ideal HTLC temperature control systems should assure effective homogeneous heating efficiency for both the mobile and the stationary phase throughout the separation column to eliminate thermal mismatch. The column temperature should be controlled precisely. If UV detection is employed, the cooling of the effluent before reaching the detector should be efficient and the temperature of the UV flow cell should be constant even during temperature-programmed elution.
Although most analytes are found to be stable under HTLC conditions, as discussed previously, analyte degradation must be monitored when HTLC is employed. For separation and analysis of biomolecules, subambient or low temperature is more appropriate because the high temperature used in HTLC can cause analyte degradation. If HTLC is used for analysis of biomolecules, ultrafast separation conditions should be considered to minimize the analyte exposure time to the harsh high-temperature conditions.
Yu Yang, Department of Chemistry, East Carolina University, Greenville, North Carolina. Please direct correspondence to yangy@mail.ecu.edu
References
(1) C.J. Dunlap, C.V. McNeff, D. Stoll, and P.W. Carr, Anal. Chem. 73, 598A–607A (2001).
(2) J. Nawrocki, C. Dunlap, A. McCormick, and P.W. Carr, J. Chromatogr., A 1028, 1–30 (2004).
(3) J. Nawrocki, C. Cunlap, J. Li, J. Zhao, C.V. McNeff, A. McCormick, and P.W. Carr, J. Chromatogr., A 1028, 31–62 (2004).
(4) T. Greibrokk, Anal. Chem. 74, 375A–378A (2002).
(5) T. Greibrokk and T. Andersen, J. Chromatogr. A 1000, 743–755 (2003).
(6) A.M. Edge, S. Shillingford, C. Smith, R. Payne, and I.D. Wilson, J. Chromatogr., A 1132, 206–210 (2006).
(7) D. Guillarme, S. Heinisch, and J.L. Rocca, J. Chromatogr., A 1052, 39–51 (2004).
(8) E. Ayano, Y. Okada, C. Sakamoto, H. Kanazawa, A. Kikuchi, and T. Okano, J. Chromatogr., A 1119, 51–57 (2006).
(9) Y. Yang, Anal. Chim. Acta 558, 7–10 (2006).
(10) Y. Yang, LCGC Eur. 16, 37–41 (2003).
(11) Y. Yang, LCGC 24 S4), 53–58 (2006).
(12) Y. Yang, J. Sep. Sci. 30, 1131–1140 (2007).
(13) C. Shih, Y. Chen, J. Xie, Q. He, and Y. Tai, J. Chromatogr., A 1111, 272–278 (2006).
(14) J.D. Thompson and P.W. Carr, Anal. Chem. 74, 1017–1023 (2002).
(15) T. Teutenberg, H.-J. Goetze, J. Tuerk, J. Ploeger, T.K. Kiffmeyer, K.G. Schmidt, W. Kohorst. T. Rohe, H.-D. Jansen, and H. Weber, J. Chromatogr., A 1114, 89–96 (2006).
(16) B. Yan, J. Zhao, J.S. Brown, J. Blackwell, and P.W. Carr, Anal. Chem. 72, 1253–1262 (2000).
(17) Y. Mao and P.W. Carr, LCGC 21, 150–167 (2003).
(18) D.R. Stoll and P.W. Carr, J. Am. Chem. Soc. 127, 5034–5035 (2005).
(19) Y. Yang, M. Belghazi, A. Lagadec, D.J. Miller, and S.B. Hawthorne, J. Chromatogr., A 810, 149–159 (1998).
(20) D.J. Miller, S.B. Hawthorne, Anal. Chem. 69, 623–627 (1997).
(21) Y. Yang, A.D. Jones, J.A. Mathis, and M.A. Francis, J. Chromatogr., A 942, 231–236 (2002).
(22) D.W.J. Hooijschuur, C.E. Klentz, and U.A.T.Brinkman, J. High Res. Chrom. 23, 309–316 (2000).
(23) Y. Yang, T. Kennedy, and T. Kondo, J. Chromatogr. Sci. 43, 518–521 (2005).
(24) C.A. Bruckner, S.T. Ecker, and R.E. Synovec, Anal. Chem. 69, 3465–3470 (1997).
(25) T.S. Kephart and P.K. Dasgupta, Talanta 56, 977–987 (2002).
(26) D. Louden, A. Handley, R. Lafont, S. Taylor, I. Sinclair, E. Lenz, T. Orton, and I.D. Wilson, Anal. Chem. 74, 288–294 (2002).
(27) R.M. Smith, O. Chienthavorn, I.D. Wilson, B. Wright, and S.D. Taylor, Anal. Chem. 71, 4493–4497 (1999).
(28) C. Smith, B.P. Jensen, I.D. Wilson, F. Abou-Shakra, and D. Crowther, Rapid Commun. Mass Spectrom. 18, 1487–1492 (2004).

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.














