Update in the Technology and Applications of Chiral Stationary Phases
An ever-increasing need for chiral separations has led to a more generic approach for screening a variety of chiral stationary phases. These new screening methodologies have been supported by new instrument development, new chiral product performance, and a new level of user knowledge. Supercritical fluid chromatography has continued to grow, supported by published applications from the separations industry. An expanded field of polysaccharide phases has been made available from a variety of sources with some unique variants of the most common cellulose and amylose derivatives.
An ever-increasing need for chiral separations has led to a more generic approach for screening a variety of chiral stationary phases. These new screening methodologies have been supported by new instrument development, new chiral product performance, and a new level of user knowledge. Supercritical fluid chromatography has continued to grow, supported by published applications from the separations industry. An expanded field of polysaccharide phases has been made available from a variety of sources with some unique variants of the most common cellulose and amylose derivatives.
In 2006, at the time of the last review (1), 80% of small molecule drugs approved by the FDA were chiral and 75% were sold as single enantiomers (2). Looking forward, it has been estimated that 200 chiral compounds will enter the development process each year. A variety of chiral technologies have expanded to meet this challenge, including asymmetric synthesis involving chiral auxiliaries, salt resolutions, chemical and biocatalysis, and chromatographic separations. Throughout these processes, versatile analytical methodologies are required. Because speed is essential, due to time and cost pressures and the growing number of projects, generic screening methods have become the preferred course of action. Generic methods are typically chromatographic separation methods employing chiral stationary phases (CSPs) that have broad selectivity capabilities. The pressure of developing an increased number of analytical methods ironically has led to a reduction in the number of suitable stationary phases tested for a particular separation and, in addition, a new view that preparative separations are in reality the best course of action for obtaining pure enantiomers instead of a method of "last resort."
Also during 2006, a major shift already was taking place in the approach to chiral separations. Blind screening of a number of chiral stationary phases was seen as the best approach to getting selectivity data in the most efficient manner. High performance liquid chromatography (HPLC) systems designed to screen from six to as many as 12 columns (PDR-Chiral, Lake Park, Florida) were infiltrating the market with a configuration of eight being the most common. Sepiatec (Berlin, Germany) and Eksigent (Dublin, California) have taken this methodology a step further in offering parallel testing of eight CSPs. In the interim, many consolidations have taken place within the pharmaceutical industry and within the chiral separations businesses, creating an even stronger concentration of effort toward greater efficiency in the separation process.

As the focus on chirality has brought the chiral separation process closer to the drug discovery platform, the need for chiral analysis increased and the requirements for even shorter method development times emerged. To respond to this growing need, the focus in HPLC centered on shorter columns and smaller particles using higher pressures and temperatures in the hope of achieving faster, more efficient analyses. Many of these approaches were not directly applicable to all chiral stationary phases. Certain of the CSPs require longer columns to obtain the necessary plate height for resolution while for other CSPs, higher temperatures adversely affected column stability. Some compromises had to be made. Temperature as an operating parameter has been studied extensively (3) especially for the CHIROBIOTIC macrocyclics glycopeptides phases (4,5). An alternative approach better suited to chiral separations was demonstrated by operating a CSP in a different mode. In liquid-phase methodology, two basic modes of operation have developed; HPLC and sub- and supercritical fluid chromatography (SFC). SFC utilizing super- and subcritical carbon dioxide modified with methanol or other solvents is seen as a potential major contributor to resolving many of the method development issues, especially relating to speed but also to environmental issues, both of major concern to the pharmaceutical and chemical industries. In addition, compared with HPLC, SFC also is considered to be a more "green" technique with limited amounts of organic solvent content in a carbon dioxide environment that also helps to make solute collection a cleaner process because the majority of the solvent (that is, carbon dioxide) merely evaporates to a gaseous state.
The limiting factor for SFC methodology centers on the polarity of the molecule. As the polarity of the molecule increases additional percentages of additives are required to promote elution and the process slows back down to conventional chromatography. Fortunately, the polarity of the majority of current molecules falls within the suitable range of normal phase chromatography and, therefore, of SFC.
Chiral Selectivity: Separation Methods
A number of different separation methodologies have been explored to date in an attempt to bring the necessary efficiency to this difficult separation problem. Capillary electrophoresis and capillary electrochromatography, while generating a large volume of published papers, have not succeeded in bringing a stable, efficient solution to the problem. Eksigent has managed to bring parallel screening together successfully with capillary chromatography. The translation, however, to conventional HPLC for the purpose of broad use or to preparative methodologies within an organization has not been established because published data has not been made available to date.
Capillary gas chromatography (GC) has served well in the analysis of chiral building blocks for evaluating chiral raw material sources (6) as chiral building blocks, but the limitations of analyte volatility and stability have limited the use of this technique. In addition, it does not have preparative capabilities. HPLC has proven to be the best methodology to deal with the diversity and complexity of the problem. We will therefore focus on the two basic modes of operation that are most utilized; HPLC and SFC.
Liquid Chromatography Methods
The majority of pharmaceutical companies are now screening routinely for chiral selectivity with column-switching systems that screen four, six, or eight chiral stationary phases on a 24-h basis in a specialized service facility. In some other companies, decentralization has occurred so that the separation activity is closer to the source. In these situations, simple "walk up" HPLC systems are available, utilizing a limited selection of columns that have been set up previously. Under these conditions, only the most difficult separation problems are sent off to the specialist. To enhance the capabilities of both the routine analyst and the centralized specialist, two fully automated, parallel column screening systems were introduced by Sepiatec and Eksigent, enabling a greater breadth of CSP choices that can be screened. In these situations, the overall efficiency and productivity for solving chiral separation problems appears to have increased fourfold. Moreover, a better outcome will be achieved in satisfying the variety of application needs in addition to what is required already for preparative purifications. Fast screening for selectivity is obviously the key.
SFC Methods
As in much of the pharmaceutical industry, consolidation has occurred in SFC as well with the acquisition of Berger Instruments, formerly part of Mettler-Toledo (Newark, Delaware), by Thar (Pittsburgh, Pennsylvania). Many demonstrations of the speed of this methodology have been published and the cost savings in preparative applications is a very large advantage. There is no doubt that this technique offers speed and very much reduced solvent costs for both the purchase and disposal of mobile phase components, but SFC has demonstrated its limitations to normal-phase separations. It is best to say that this technique is very complementary to standard HPLC operation. That said, the cellulose and amylose phases from Daicel (West Chester, Pennsylvania), including the CHIRACEL OD and OJ and CHIRALPAK AD and AS, have played a major role in the chiral separation of neutral and less polar molecules. The new bonded CHIRALPAK IA, IB, and IC phases are now being promoted to replace the former coated CSPs for their long-term stability and solvent diversity. For the resolution of more polar racemates, the Supelco CSPs CHIROBIOTIC TAG and the CYCLOBOND DNP and DMP as well as the polymeric phases P-CAP and P-CAP-DP have been complementary. The Regis Technologies (Morton Grove, Illinois) phases, primarily the WHELK-O1, WHELK-O2, and ULMO, also have played a major role in the successful application of the SFC technique.
Advances in Technology Currently in Use
Polysaccharide chiral stationary phases: In 2006 Chiral Technologies, Inc. (Daicel) introduced the CHIRALPAK IA phase as the bonded (immobilized) 3,5-dimethylphenyl carbamate derivative of amylose, corresponding to the coated version CHIRALPAK AD. The second in this series of bonded polysaccharide phases, designated CHIRALPAK I B, was introduced as the 3,5-dimethylphenyl carbamate derivative of cellulose that is trademarked as CHIRALCEL OD. Recently, a new bonded derivative has been introduced designated the CHIRALPAK I C that is described as a 3,5-dichloro-phenyl carbamate derivative of cellulose. Figure 1 demonstrates the suitability of these new bonded phases for SFC applications. It has been announced that these bonded phases are now available in 3-, 5-, and 20-μm particle sizes. The stated benefits of the bonded versions have been the extended solvent flexibility and extended durability over the coated versions. Applications on these phases demonstrate the use of solvents like methylene chloride, methyl-tert-butyl ether, and chloroform. At the International Symposium on Chirality (ISCD) 2005 meeting in Parma, Italy, it was reported that the method development process is quite different from the coated versions. It appears that these new bonded versions could be relied on for the resolution of existing problems with coated versions rather than as a direct replacement (7).
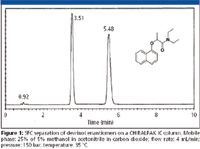
Figure 1
Several companies now have introduced similar products in this area for these most widely used polysaccharide-family of coated chiral stationary phases for HPLC enantioseparations. Many companies started with the typical 3,5-dimethylphenyl–derivatized cellulose and now have added the same derivative for the amylose. These are presented here in no particular order or preference.
Macherey-Nagel (Berlin, Germany) has introduced NUCLEOCEL DELTA, the 3,5-dimethylphenyl carbamate derivative of cellulose typically used for normal-phase separations, and NUCLEOCEL DELTA-RP, for reversed-phase use. These phases are coated on silica and offered in 5- and 10-μm particle sizes for analytical and preparative use.
Sepaserve (Muenster, Germany) introduced their own special derivatized version of these popular polysaccharides, a halogen-methyl substituted cellulose called Sepapak-2. The company has now added the amylose base, similarily derivatized, called Sepapak-3.
AECS-QuikPrep (South Wales, UK) has introduced Quik Prep characterized as a nonclone but a better match between the polarity of many target active pharmaceutical ingredients and eluent polarity coated on macroporous silica for both the cellulose version called CelCoat RBC-1 and the amylose version similarly described, designated AmylCoat. The company also claims to have a near-clone version. For the former coated versions, it is recommended to use a gradient for methods development, as the typical method development strategy will not work.
Akzo Nobel (Bohus, Sweden) has utilized its position as a manufacturer of high-quality silica to introduce versions of the amylose designated Kromasil AmyCoat, and cellulose phases designated Kromasil CelluCoat. The silica base is specified as an in-house-developed super-wide pore matrix coated with the typical tris-(3,5-dimethylphenyl carbamate) derivatives of amylose and cellulose, respectively. They are reported to tolerate pressures up to 400 bar. The availability in particle sizes from 3 to 25 μm offers efficiency and speed for analytical and durability for preparative applications. The speed advantage of 3 μm can be seen in Figure 2.
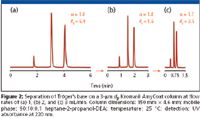
Figure 2
The CHIROBIOTIC, macrocycolic glycopeptides phases, patented products of Advanced Separation Technologies, Inc. (ASTEC, Whippany, New Jersey) have now been incorporated fully into the chromatography section of Supelco (Bellefonte, Pennsylvania) as a result of the purchase of ASTEC in 2006 by Sigma-Aldrich. The product line has been through a vigorous standardized manufacturing procedure at Supelco and is now available around the world through the Sigma-Aldrich distribution network. A major change, however, did occur in the Supelco method development screening protocol utilizing the CHIROBIOTIC products. In this change, the CHIROBIOTIC V2 has been shown to have a significant advantage over the CHIROBIOTIC V for chiral amines of all types, especially in the polar ionic mode, Figure 3, a non-aqueous, normal phase system and to a lesser extent in the reversed-phase mode. The addition of the CYCLOBOND DNP (π-acid) and the CYCLOBOND DMP (π-base) to the Supelco method development protocol also has expanded greatly the selectivity hits for a large number of racemates not separated by other CSPs. All validated separations have been accomplished in LC–MS compatible mobile phases. Supelco has expanded their method development service and is building a new facility to accommodate the expanded chiral separation capabilities. A further advantage of the Supelco acquisition of Astec has been the availability of other sample preparation technologies like solid-phase extraction (SPE), solid-phase microextraction (SPME), and a hybrid SPE–molecular imprinted polymer (MIP) to further enhance chiral assay development.

Figure 3
New Concepts in Chiral Stationary Phases
Truly new technologies that have been cited in the commercial arena have been sparse. The use of nanotechnology to structure chiral cavities has disappeared (1). However, ZirChrom (Anoka, Minnesota) has presented information on the use of zirconia as a versatile substrate for chiral stationary phase development. The company has promoted a bidentate anchor, pamidronic acid, as the key to improved stability and regenerability. Various chiral selectors were evaluated including (S)-DNB-L-leucine, (S)-DNB-L-phenylglycine and (S)-{1-(1-naphthyl)ethyl}succinamic acid. The most interesting fact is the ability to regenerate a new stationary phase from the base and yet show stability of the useable product at pH 2 and pH 8. They also took the interesting step of synthesizing a cellulose-coated zirconia stationary phase anchored with undecyphosphonic acid, which also could be regenerated. The 3,5-dimethylphenyl modified cellulose has good selectivity compared to a commercial silica-based column in typical 90:10 hexane–isopropanol but lower retention values undoubtedly due to lower ligand loading.
Conclusions
Routine chiral separations have taken on a generic approach and are now possible given the advances in both product availability, performance, and user knowledge. There are still chiral separations that beg solutions, but new and innovative technologies have been slow to develop. Through new screening protocols, great gains have been made in the speed and number of chiral separations that are accomplished each day. Expanded sources for the most popular chiral stationary phases, the polysaccharides, are now available. SFC has made new gains in both the analytical and preparative area of chiral separations. Automation, as in most areas of drug discovery, has found its way into chiral separations as witnessed by recent instrument introductions.
Thomas E. Beesley, Sigma-Aldrich/Supelco, Bellefonte, Pennsylvania. Please direct correspondence to astectebeesley@aol.com
References
(1) T.E. Beesley, LCGC 24(S4), 28–31 (2006).
(2) Centering on Chirality, C&E News, August 6, 2007.
(3) R.P.W.Scott, The Effect of Temperature and the Composition of Non-Associating Binary Solvent Mixtures on Solute Selectivity in Liquid Chromatography (The Royal Society of Chemistry, 1999).
(4) A. Berthod, B.L. He, and T.E. Beesley, J. Chromatgr., A 1060, 205–214 (2004).
(5) P.A., E. Vekes, and D.W. Armstrong, J. Chromatogr., A. 958, 89–107 (2002).
(6) J. Liq. Chromatog. Rel. Technol. 28, 1-40 (2005).
(7) S. Andersson et al., "Differences in the Chiral Recognition Abilities of Coated vs Immobilized Polysaccharide Chiral Stationary Phases and Their Application in Preparative Chiral Separations," ISCD-17, Parma, Italy, paper 029.

Determining Enhanced Sensitivity to Odors due to Anxiety-Associated Chemosignals with GC
May 8th 2025Based on their hypothesis that smelling anxiety chemosignals can, like visual anxiety induction, lead to an increase in odor sensitivity, a joint study between the University of Erlangen-Nuremberg (Erlangen, Germany) and the Fraunhofer Institute for Process Engineering and Packaging (Freising, Germany) combined behavioral experiments, odor profile analysis by a trained panel, and instrumental analysis of odorants (gas chromatography-olfactometry) and volatiles (gas chromatography-mass spectrometry).
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












