HPLC Separation of 30 Perfluorinated Compounds and Isomers Using a Pentafluorophenyl Reverse Phase Column
Perfluorinated compounds (PFC) are considered as emerging contaminants that attract high interest from the international scientific community.
Luisa Pereira1 , Hanane Kadar2 , Bruno Veyrand2 , Emmanuelle Bichon2 , Fabrice Monteau2 , Jean-Philippe Antignac2 , and Bruno Le Bizec2 , 1 Thermo Fisher Scientific and 2 Laboratoire d'Etude des Résidus et Contaminants dans les Aliments (LABERCA)
Perfluorinated compounds (PFC) are considered as emerging contaminants that attract high interest from the international scientific community. They are present in a large amount of commercialized products mainly as an anti-sticking or amphoteric material (carpet, clothes, fire-fighting-foams, detergents, etc.). They can be consequently released into the environment during any part of their life cycle, from production to final use. Some toxicological effects have been reported for PFCs (for instance hepatotoxicity and endocrine disruption), but there are still no regulations regarding these compounds in Europe. PFCs can be synthesized by two different processes, namely electrochemical fluoration or telomerisation. The latter process leads to either linear or branched isomers. Thus, to quantify the linear isomers of perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA), which are the main degradation products of PFC, their efficient separation is required. The aim of this work is to separate thirty perfluorinated compounds, including isomers of PFOA and PFOS, in a short time analysis with the Thermo Scientific Hypersil GOLD PFP column.
Experimental
PFCs analysis was performed using a binary HPLC pump coupled with a triple quadrupole MS system. Separation was achieved using a Hypersil GOLD™ PFP 1.9 µm 100 × 2.1 mm column. A mobile phase of aqueous ammonium acetate 20 mM (A) and methanol (B) at 0.45 mL/min was used with the following gradient: 20% B for 0.9 min, then up to 70% B by 1.8 min and 80% B by 5.3 min; kept at 80% B for 1.4 min and then increased to 100% B by 8 min and kept at this concentration up to 10.7 min. Reverted back to 20% B by 12 min and kept at this concentration until 20 min. Column temperature was kept at 55 °C and a 10 µL injection of 0.02 ng/µL standard solution in A:B (80:20) was performed. Detection was in MRM.
Results
Elution using the Hypersil GOLD PFP column was optimized with an isocratic plateau from 5.3 to 7 min to help separate the majority of the compounds (Figure 1).
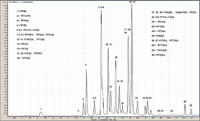
Figure 1: Separation of 30 perfl uorinated compounds on penta-fl uorophenyl column (Hypersil GOLD PFP).Moreover, the addition of ammonium acetate in the mobile phase allows for improved chromatographic separation. Using the same gradient, a better separation was observed on the pentafluorophenyl phase compared to an alkyl chain phase (data not shown). The superior selectivity of the perfluorinated phase can be explained by the capability of the pentafluorophenyl phase to provide to dipole-dipole, p-p, charge transfer, and ion-exchange interactions with the analytes. Furthermore, PFOA isomers were found to be equally separated on both columns (Figure 2).
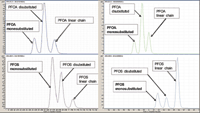
Figure 2: Comparison of isomers' separation on Hypersil GOLD PFP phase on the left and on Hypersil GOLD phase on the right.
Conclusion
A method has been developed for the separation and LC–MS-MS analysis of 30 PFCs in 12 min. Two phases were compared, a classical reversed-phase chemistry and a perfluorinated phase. On the basis of the same elution gradient, fewer coelutions were observed with the perfluorinated phase. Finally, this method will be applied to analyze perfluorinated compounds in fish.
Thermo Fisher Scientific
Tudor Road, Runcorn, Cheshire WA7 1TA, UK
tel. + 44 (0) 1928 534324
Website: www.thermoscientific.com/columns

Separation of Ultra-Short and Long Chain PFAS Compounds Using a Positive Charge Surface Column
December 11th 2024A separation of ultra-short and long chain PFAS (C1-C18) is performed on a HALO®PCS Phenyl-Hexyl column along with a HALO®PFAS Delay column which demonstrates excellent retention for both hydrophilic and hydrophobic analytes.















