The Essential Guide to Electron Ionization in GC–MS
The “must know” details of which all electron ionization (EI) gas chromatography–mass spectrometry (GC–MS) users should be aware.
The “must know” details of which all electron ionization (EI) gas chromatography–mass spectrometry (GC–MS) users should be aware.
In an electron ionization (EI) source, analyte ions in the gas phase encounter a stream of thermionic electrons with 70 electron volts (eV) of energy, emitted from the surface of a heated metal filament. The energy from these electrons is transferred, in part, to an analyte (no collisions are involved!), which causes the ejection of an electron from, and atom within, the analyte molecule, forming a radical (odd electron) cationic species:
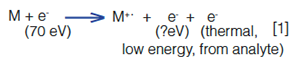
The amount of energy required to remove an electron from smaller organic molecules typically ranges from 8 to 12 eV, and any excess energy imparted by the ionizing electron may cause bond breakage and the formation of fragments (note that not all of the remaining electron energy is necessarily transferred to the analyte molecule).
The site of ionization is perhaps the first consideration when attempting to better understand the ionization process, and, in general, the following series indicates the energy required (and therefore favourability) of ionization site:
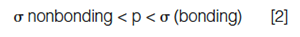
So, if an analyte were to contain a heteroatom, for example, containing nonbonding electrons, one might first begin to elucidate the spectrum obtained by assuming that the charge is cited on the heteroatom, and so on.
In electron ionization mass spectrometry, the intensity of the spectral signal for a molecular ion or fragment is dependant upon the energetic favourability of that species being formed, which is usually closely linked to the ability of the resulting ion to stabilize the charge which it carries, as well as the stability of the radical or molecular product (that is, the stability of all products must be considered). For this reason, highly unsaturated or aromatic species, which are able to stabilize charge, tend to have their most intense fragments at the higher molecular weight end of the spectrum (the right-hand end), and alkane species, which are less able to stabilize the charge on the molecular ion, tend to fragment more readily, and the most intense fragments will lie to the lower molecular weight end (the left-hand end of the spectrum).
Identifying the molecular ion within the spectrum is important, as it provides the molecular weight of the analyte, which is obviously very helpful for analyte identification. If a very weak molecular ion is suspected, one may reduce the energy of the ionizing electrons, from 70 eV down to around 25 eV, before signal intensity becomes too weak to distinguish from noise. This tends to promote the intensity of the molecular ion, and helps us confirm the suspected molecular ion.
When considering the nature of the fragments formed, and thus the chemical nature of the analyte, there are some “typical” mechanisms and pathways with which fragments
are formed, typically to stabilize the charge on the analyte molecule, see Figure 1.
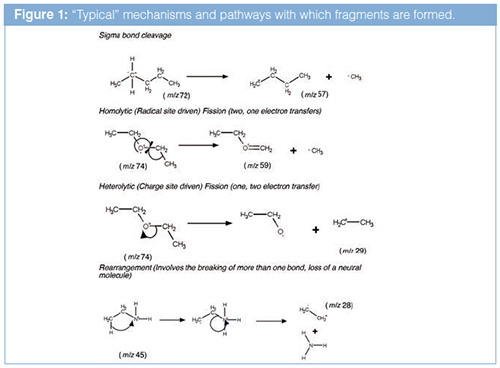
Remember that only the charge products are seen within the mass spectrometer, as we cannot guide the neutral species through the mass spectrometer towards the detector.
There are many more “tools” that can be used in spectral interpretation and those described above represent just some the fundamental considerations.
For further information see: https://www.chromacademy.com/mass-spec-training.html

Best of the Week: Food Analysis, Chemical Migration in Plastic Bottles, STEM Researcher of the Year
December 20th 2024Top articles published this week include the launch of our “From Lab to Table” content series, a Q&A interview about using liquid chromatography–high-resolution mass spectrometry (LC–HRMS) to assess chemical hazards in plastic bottles, and a piece recognizing Brett Paull for being named Tasmanian STEM Researcher of the Year.
Using LC-MS/MS to Measure Testosterone in Dried Blood Spots
December 19th 2024Testosterone measurements are typically performed using serum or plasma, but this presents several logistical challenges, especially for sample collection, storage, and transport. In a recently published article, Yehudah Gruenstein of the University of Miami explored key insights gained from dried blood spot assay validation for testosterone measurement.
Determination of Pharmaceuticals by Capillary HPLC-MS/MS (Dec 2024)
December 19th 2024This application note demonstrates the use of a compact portable capillary liquid chromatograph, the Axcend Focus LC, coupled to an Agilent Ultivo triple quadrupole mass spectrometer for quantitative analysis of pharmaceutical drugs in model aqueous samples.
