New HPLC Systems and Related Products Introduced in 2018–2019: A Brief Review
LCGC Europe
This instalment describes high performance liquid chromatography (HPLC), mass spectrometry (MS), and related products introduced at Pittcon 2019 and during the year prior. It reviews new HPLC and MS systems, modules, chromatography data systems (CDS), and other related software and summarizes their significant features and user benefits. A brief description of instrumentation trends and the current market is also included.
This instalment describes high performance liquid chromatography (HPLC), mass spectrometry (MS), and related products introduced at Pittcon 2019 and during the year prior. It reviews new HPLC and MS systems, modules, chromatography data systems (CDS), and other related software and summarizes their significant features and user benefits. A brief description of instrumentation trends and the current market is also included.
This instalment marks my 7th anniversary as a columnist for LCGC Europe’s “Perspectives in Modern HPLC” column. My first contribution covered new high performance liquid chromatography (HPLC) product introductions in 2013. And though even then our coverage of new products reached well beyond Pittcon, the event remains a major event in analytical chemistry and one where many new products continue to be launched. And for that reason, we produce this review after Pittcon each year, and include in our coverage the sense of the field that we get from attending.
At the Pittcon 2013 meeting, I recall the country was still feeling the aftermath of the 2008 financial crisis and the host city, Philadelphia, was not immune to its effects. Much has changed in the years since. The economy is in much better shape, and Philadelphia is thriving, undergoing an urban renewal with many new buildings, hotels, and upscale restaurants springing up in Center City.
Philadelphia is an appropriate conference site for conferences in the Northeast. The city has many industrial and pharmaceutical research centres, as well as plenty of notable universities such as the University of Pennsylvania, Drexel University, and Temple University. Philadelphia’s proximity to major cities also helps to make it an excellent site; it is within a two- or three-hour drive of both New York City and Washington D.C. The Pennsylvania Convention Center is a few blocks from Independence Hall, the famous site where the U.S. founding fathers signed the Declaration of Independence in 1776 and later the Constitution of the United States in 1787. Besides the historical richness of the location, the convention centre is across the street from the Reading Terminal Market and Philadelphia’s Chinatown, where one finds many ethnic eateries offering great food at reasonable prices.
Pittcon 2019 boasted ~13,000 attendees from industry, academia, and government agencies representing over 90 countries. There were 200 plus technical sessions, including plenary lectures, invited, contributed, and award symposia, workshops, posters, and networking sessions, as well as ~90 short courses, and a huge threeâday exposition with more than 700 vendors.
Appropriate to the historical significance of the city, Dr. Fenella G. France, the Chief of the Preservation Research and Testing Division at the Library of Congress, gave the plenary lecture on “Preserving and Revealing History-Challenges of a Cultural Heritage Scientist”. In the Wallace H. Coulter lecture, Nobel Laureate Professor Fraser Stoddart discussed a “New World of Wonders on Materials Beyond Cyclodextrins”. Other presentations by award winners in separation sciences were delivered by Weihong Tan of the University of Florida, by the founders of Supelco, Walter Supina and Nicholas Pelick, and by Peter Schoenmakers of the University of Amsterdam, Milos Novotny of Indiana University, and Ken Broeckhoven of Vrije Universiteit of Brussel.
Trends in HPLC and Mass Spectrometry (MS) Products and the Current Market
Before describing any new products introduced over the last year, I will start with a brief discussion of modern trends in HPLC and MS instrumentation, and the current market for them. The market for HPLC and MS instruments was measured at ~$10 billion in 2018. This market size estimate appears surprisingly low, especially when considering the impact that these instruments have in driving scientific discovery (1–3).
Current Market for HPLC Systems
Four major HPLC manufacturers, Waters, Agilent, Thermo Fisher Scientific, and Shimadzu, have been consistently responsible for more than 80% of the global market in recent years.
Waters Corporation has been the HPLC market leader since the 1970s. They were the first to commercialize ultrahigh-pressure liquid chromatography (UHPLC) technology in 2004 with their Acquity UPLC instrument (4–6). Their success has persisted with their newer UHPLC systems such as the Acquity H-Class, I-Class, M-Class, and the Acquity Arc systems. Moreover, their Empower CDS has enjoyed widespread acceptance by regulators as demonstrated by its ubiquity in pharmaceutical laboratories.
Agilent’s HPLC systems are popular in research laboratories thanks to their modular 1100, 1200, 1260, and 1290 series HPLC product lines. Agilent’s current product line consists of the secondâgeneration UHPLC Infinity II series, which includes the 1290 binary and quaternary, the 1260, and 1220 series. Recently revamped versions of Agilent’s OpenLab CDS greatly improved data handling and included regulatory compliance features that have enhanced its competitiveness in quality control laboratories.
Thermo Fisher Scientific, already well known for its innovative MS products, became a serious competitor in the chromatography market following the acquisition of Dionex in 2011. The acquisition added ion chromatography, the Chromeleon CDS, and the Ultimate 3000 UHPLC systems to its portfolio. The introduction of the Vanquish UHPLC in 2014 further bolstered Thermo’s presence in chromatography.
Shimadzu offers the Prominence and Nexera Series, two wellâdeveloped, integrated, and modular HPLC and UHPLC product lines covering microflow through preparative purification applications. These products are supplemented by preconfigured systems for specific applications (such as the Cannabis Analyzer) and automated sample preparation modules (such as the Clinical Laboratory Automation Module, or CLAM-2030, for
LC–MS). The recent introductions of its supercritical fluid chromatography (SFC) system that includes preparative SFC and a supercritical fluid extraction system, and a more complete MS product line, add to Shimadzu’s presence in the food, environmental, pharmaceutical, quality control, and industrial markets.
Other HPLC Companies: Other global providers of HPLC instruments include Danaher (Sciex, Eksigent), Jasco, Knauer, Hitachi, and PerkinElmer, as well as producers of HPLC modules such as Metrohm, Scientific Systems (Teledyne Isco), Bischoff, LEAP Technologies, Showa Denko, Sonntek, Spark Holland, Tosoh Bioscience, and Wyatt Technologies. Additionally, a few new HPLC manufacturers have seen some localized success in recent years, particularly in academia. New entries in the HPLC market find it difficult to compete with the major brands but can penetrate the market with niche instruments, such as the portable LC system (Focus) launched by Axcend in 2018.
Mass Spectrometry (MS): Mass spectrometers separate analytes by their mass-to-charge ratio (m/z) in a high vacuum and offer unprecedented analytical sensitivity and selectivity for ionizable compounds. HPLC–MS is arguably the most powerful analytical technique in scientific discovery, particularly in biosciences (3–4). Major types of MS include the following:
- Magnetic sector: the oldest type of MS system, using a permanent magnet; primarily used in gas analyzers.
- Single quadrupole: the most common type of MS instrument, with unit mass resolution useful for peak identification and confirmation.
- Triple quadrupole or tandem MS: With two single quadrupoles in series with a middle radio frequency-only quadrupole for collision-induced fragmentation, triple quadrupole or tandem MS instruments use multiple reaction monitoring as the gold standard for trace quantitation of complex samples in bioanalytical and multiresidue assays.
- Ion trap: a compact type of MS system useful for structure elucidation by trapping analyte ions and performing sequential fragmentation.
- Time-of-flight (TOF): a highâresolution type of MS system using a long flight tube that differentiates ions by measuring their times of flight. A reflectron is often used to extend the flight path (and to reduce the overall instrument footprint).
- Fourier-transform ion cyclotron resonance (FT-ICR): a type of MS offering very high resolution and mass accuracy based on the cyclotron frequency of the ions in a fixed magnetic field cooled by liquid helium and nitrogen.
- Orbital ion trap: an elliptical ion trap instrument that utilizes a Fourier transform algorithm to yield very high mass resolution for qualitative and quantitative analysis. This type of instrument is more compact than FT-ICR and is a proprietary product marketed solely by Thermo Scientific.
- Hybrid and tribrid: MS instruments combining two or more types of MS such as QTOF or Q-orbital trap-ion trap are particularly useful for structure elucidation and the analysis of complex samples (proteomics) and biomolecules.
It is not surprising that the top four HPLC manufacturers are also successful providers of MS instruments. Waters entered the MS market via their acquisition of Micromass in 1997 and continues to offer a competitive line of MS instruments. Agilent (formerly Hewlett-Packard) was an early manufacturer of singleâquadrupole MS instruments for gas chromatography (GC). They offer a wide choice of single-quadrupole, triple-quadrupole, TOF, and QTOF-MS instruments. Finnigan Instruments, acquired by Thermo Scientific in 1990, was the first maker of single-quadrupole and ion-trap instruments. Thermo Scientific is currently a leading manufacturer of diversified MS equipment including single-quadrupole, ion-trap, orbitalâion trap, FT-ICR, and various hybrid and tribrid systems. Shimadzu has recently expanded its MS offerings of single-quadrupole and tripleâquadrupole systems to include QTOF equipment.
Sciex (a subsidiary of Danaher) was the first company to introduce triple-quadrupole systems and continues to dominate the market for bioanalytical analysis, with instruments like the 6500+. Bruker, the leader in nuclear magnetic resonance (NMR) instruments, also supplies FT-MS, ion-trap, triple-quadrupole, TOF, QTOF, and ion mobility MS systems. Other MS manufacturers include Advion, Hitachi, Jeol, LECO, and PerkinElmer. Additionally, there have been several recent entries of compact and transportable MS instruments from 1st Detect, 908 Devices, and Microsaic Systems (3).
Emerging Trends for HPLC and MS Systems: The most important development in HPLC was the introduction of UHPLC instruments. Compared to HPLC, UHPLC is capable of higher operating pressures and lower system dispersion (5–7) used in conjunction with sub-2-μm particle columns. The debut of the first commercialized UHPLC system in 2004 spurred on waves of UHPLC instrument introductions by other major manufacturers. Current HPLC systems available include UHPLC (>15,000 psi or 1000 bar), conventional HPLC (<6000 psi or 400 bar), intermediary (9000–12000 psi or 600–900 bar), dual-path systems (Acquity Arc, Thermo Vanquish Flex, and Duo), and systems preconfigured for specific workflows or applications such as method development, two-dimensional LC (2D-LC), and cannabis analysis).
MS is currently undergoing a boom in development fueled by the increasing demand from the pharmaceutical, biotechnology, industrial, environmental, food,
and clinical diagnostics industries. New instruments are trending towards more compact laboratory systems such as the Waters Acquity QDa, Advion expression CMS, and Agilent Ultivo. Recent trends include highly portable point-of-use instruments, such as those from 1st Detect and 908 Devices, and high-resolution hybrids or tribrids for accurate mass analysis of complex mixtures, as afforded by Thermo’s Orbitrap, or QTOFs by many manufacturers.
New HPLC, MS, and CDS Products Introduced in 2018–2019
Although new introductions of HPLC systems appear to be slowing down, manufacturers are turning their attention to tailored applications and sample preparation systems, particularly for LC–MS.
Table 1 lists new HPLC, MS, and CDS products, in alphabetical order by supplier name, introduced at Pittcon 2019 or in the prior year, followed by descriptions of and commentaries about each product.
New HPLC and UHPLC Systems and Line Extensions
New UHPLC systems introductions have slowed while manufacturers appear to be focusing on LC–MS line extensions for specific applications, such as clinical diagnostics, and sample preparation.
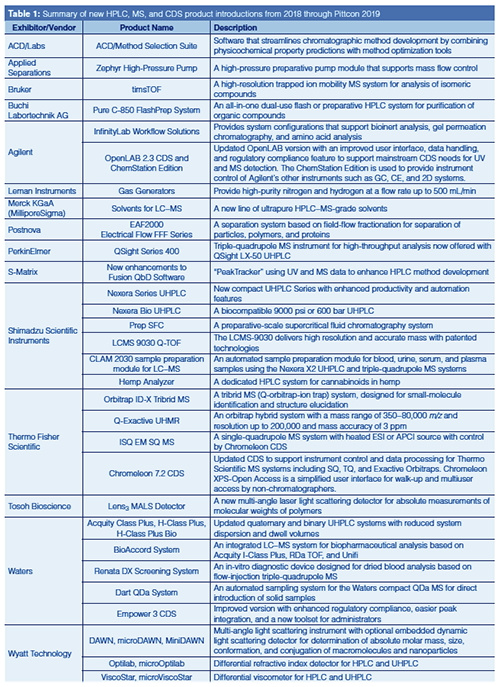
Agilent is offering several new Infinity-Lab Workflow Solutions to support bioinert analysis, gel permeation chromatography (GPC), and amino acid analysis. Agilent’s 1260 Infinity II Bio-Inert system is a titanium-based system suited for biomolecule analysis. The 1260 Infinity II Multi Detector GPC system is offered with an optional viscometer, refractive index, or light scattering detectors for organic polymers. Agilent’s Amino Acid Analysis (AAA) system uses automated precolumn derivatization with ortho-phthaldialdehyde (OPA) and fluorenylmethyloxycarbonyl chloride (FMOC) reagents and UV or fluorescence detection. An example chromatogram for the analysis of both primary and secondary amino acids in a cell culture media is shown in Figure 1 (8).
Buchi Labortechnik AG has introduced the Pure C-850 FlashPrep System, an all-in-one, dual-use flash or prep HPLC system for purification of organic compounds. This system includes both hardware and dedicated software for purification projects up to 100 mL/min and 4300 psi or 300 bar with UV or evaporative light-scattering (ELSD) detection.
Shimadzu Scientific Instruments made a significant impact at Pittcon 2019 with the introduction of a new compact Nexera Series UHPLC system with higher productivity and performance as well as automation features such as auto startup and shutdown, auto diagnostics and recovery, and mobile phase monitoring. The system is capable of injecting a sample every 7 s and can accommodate ~17,000 samples with its new plate changer. Key components of the Nexera UHPLC series include the mobile phase monitor mentioned above, the SPD-40, SPD-40V, or SPD-M40 absorbance detector, the LC-40 series solvent delivery unit, the SILâ40 series autosampler, and a new slim-line column oven.
Shimadzu also introduced the Nexera Bio UHPLC (9000 psi or 600 bar). This system features inert materials resistant to high-salt mobile phases, such as a carbon-coated pump head, gold-plated ferrules, stainless steel-clad PEEK tubing, and a ceramic injection needle.
Shimadzu also introduced the Hemp Analyzer, a dedicated HPLC platform dedicated for quantitative analysis of cannabinoid content in hemp. This system includes hardware, software, consumables, and application notes featuring three proven methods dedicated to cannabinoid analysis in hemp.
Waters updated its quaternary and binary Acquity UPLC systems (I-Class Plus, H-Class Plus, and H-Class Plus Bio) with a reduced system dispersion of 7 to 12 μL and a diminished dwell volume of 75 to 400 μL. Furthermore, improvements to the system in the solvent degasser, in sample heating and cooling, as well as in novel sampling needle surface treatments, have greatly improved the analytical performance of these systems.
Waters also introduced the BioAccord System, an integrated LC–MS system for biopharmaceutical analysis based on the Acquity I-Class Plus and the new Acquity RDa TOF-MS system (7000 amu and mass resolution of 10,000). The BioAccord is capable of automated workflows for intact mass and subunit analysis, peptide mapping, and released glycan assays. The system provides a new level of user experience featuring a one-button start-up for power on, pump down, and to initial system setup for any trained chromatographer to generate accurate mass spectrometry data (9). This system is designed to use mass spectrometry data and informatics (Waters Unify Scientific Information System) to simplify the characterization of complex biopharmaceuticals for development and quality control laboratories.
New HPLC Modules
Applied Separations’ Zephyr high-pressure pump is a unique preparative pump module that supports mass flow control capable of 330 mL/min flow at a pressure of up to 900 bar for isocratic or multistep gradient operation.
Tosoh Bioscience introduced the Lens3 MALS detector, a new multiangle laser light scattering detector compatible with HPLC and UHPLC for absolute measurements of molecular weights of polymers based on the radii of gyrations of particles in the range of 2 to 50 nm. This detector integrates the best of both MALS and low-angle light scattering (LALS).
Wyatt Technology introduced DAWN, a new multi-angle light scattering instrument with optional embedded dynamic light scattering detector for determination of absolute molar mass, size, conformation, and conjugation of macromolecules (proteins and polymers) and nanoparticles. DAWN is configured for HPLC whereas microDAWN is the version used for UHPLC. MiniDAWN is used for characterization of macromolecules and nanoparticles up to 50 nm in radius. Wyatt also introduced Optilab and ViscoStar, a differential refractive index detector and a differential viscometer, respectively, for HPLC. Both detectors are available as micro versions for UHPLC.
New Mass Spectrometers (MS)
Bruker introduced a high-resolution trapped ion mobility MS instrument for analysis of isomeric compounds with a mass resolution of ~200. Ion mobility MS is particularly powerful when used in conjunction with a high-accuracy MS system for characterization of complex samples containing isomeric sugars and lipids.
The PerkinElmer QSight 400 series is a high-sensitivity triple-quadrupole LC–MS system with StayClean and dual source (electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) technology for robust highâthroughput analysis. It is offered with PerkinElmer’s QSight LX-50 UHPLC instrument with a binary pump (18,000 psi or 1250 bar), a dual-needle autosampler, and column oven.
Shimadzu Scientific Instruments is aggressively increasing its MS product portfolio and now offers a new LC–MS 9030 QTOF instrument that uses patented technologies to deliver both high resolution and accurate mass. Innovations include high-efficiency ion guides, proprietary UFgrating, iRefTOF, and UF-FlightTube technologies. Mass ranges are 10–2000 amu for the quadrupole and 10–40,000 amu for TOF. Mass resolutions are 0.8 u and 30,000 full width at half maximum (FWHM), with a mass accuracy of <1 ppm.
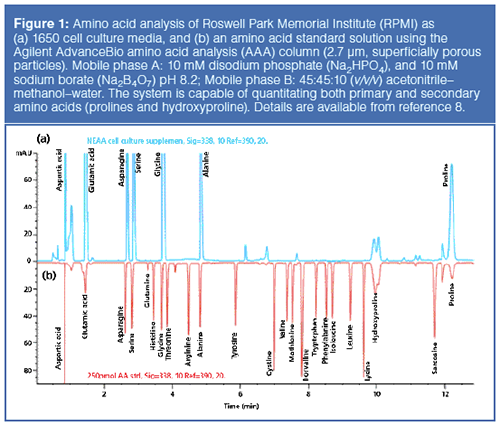
Shimadzu is also stepping up its game in the Clinical Laboratory Automation Module by offering the CLAM-2030 for LC–MS. The CLAMâ2030 is a fully automated sample preparation module for Shimadzu’s Nexera X2 UHPLC instruments and family of tripleâquadrupole MS instruments (the 8060, 8050, 8045, and 8040) for blood, urine, serum, and plasma samples. Supported functions include dispensing of samples and reagent, derivatization, stirring, filtering, heating, and sample transfer to autosamplers. An optional module configuration is available for automated toxicological screening that includes supported protocols for a 161-analytes panel. Figure 2 shows an automated workflow schematics for the CLAM-2030 in the analysis of serums with supported functionalities against that of a traditional manual workflow (10).
Thermo Scientific introduced the Orbitrap ID-X Tribrid MS consisting of a quadrupole (50–2000 amu), an orbital ion trap (up to 500,000 mass resolution and a scan rate of 30 Hz), and a dual-cell linear ion trap designed for small-molecule identification and structure elucidation.
Thermo Scientific also introduced the Q-Exactive UHMR, an orbital ion trap hybrid system with a mass range of 350–80,000 m/z, a mass resolution to 200,000, a scan rate of 12 Hz, and a mass accuracy of 3 ppm.
In addition, Thermo Scientific introduced the ISQ EM singleâquadrupole mass spectrometer with a heated ESI, APCI, or a dual source. It has an extended mass range of 10–2000 amu, a scan rate of 20,000 Da/s, and mass accuracy of <0.1 Da. Control and data handling are by the Chromeleon 7.2 CDS.
Waters introduced the Renata DX Screening System, an in-vitro diagnostic device designed for dried blood analysis based on flowâinjection MS/MS. The Renata DX incorporates the XevoTQD IVD MS, the Acquity UPLC I-Class IVD Binary Solvent Manager, and the 3777C IVD Sample Manager with appropriate MS data and application software. The RenataDX Screening System is manufactured as a U.S. FDA Class I medical device.
The Waters Dart QDa System is an automated sampling system for the Waters compact QDa singleâquadrupole mass spectrometer using a direct analysis in real time (DART) ion source for direct introduction of solid samples. It can perform rapid fingerprinting of foods and food ingredients and verify sample authenticity and adulteration.
New Chromatography Data Systems (CDS)
Agilent launched the new OpenLAB CDS in 2015 with an improved user interface, data handling, and regulatory compliance features required in pharmaceutical, food, and environmental laboratories. The current version of OpenLAB 2.3 supports additional LC and LC–MS functionalities (MS peak purity and diode array data tools), advanced reporting, e-signature capabilities, and direct connections to enterprise content management (OpenLAB 3 ECM for multivendor connectivity), laboratory information management systems (LIMS), using a sample scheduler, and electronic laboratory notebooks (ELN). The latest ChemStation Edition provides specific instrument control of Agilent’s other instruments such as GC, capillary electrophoresis (CE), and 2D-LC systems.
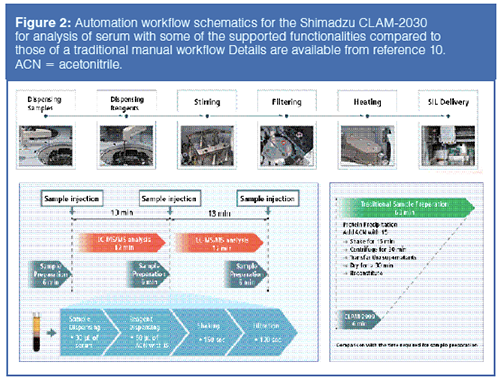
Thermo Scientific’s Chromeleon 7.2 CDS now supports instrument control and data processing for Thermo Scientific’s LC–MS and GC–MS systems for singleâquadruple, tripleâquadrupole, and Exactive Series Orbitrap systems. It supports infrastructure as a service (IaaS) cloud deployment, reducing the resources needed for training and laboratory operation. This CDS offers an extensive toolset for enhanced regulatory compliance, and automated workflow solutions support in a global network. Figure 3 shows a screenshot of the Chromeleon 7.2 displaying total ion chromatograms (TIC) and mass spectral plots. Chromeleon XPS Open Access is a simplified user interface for walk-up and multiuser access by non-chromatographers. The Waters Empower 3 CDS has been updated with enhanced regulatory compliance features, an easier peak integration algorithm, and new configuration tools for system administrators.
HPLC Method Development Software
ACD/Labs introduced the ACD/Method Selection Suite, which streamlines HPLC and UHPLC method development by combining physicochemical property predictions with method optimization tools to define better starting conditions, refine key separation parameters, and estimate retention times.
S-Matrix has introduced two new capabilities for its Fusion QbD HPLC method development software: PeakTracker and Rs–Map Response. PeakTracker automates, optimizes, and simplifies the use of photodiode array UV and MS data for LC and LC–MS method development. The “Rs–Map Response” feature uses the retention time and peak shape parameter modelling technologies to predict United States Pharmacopeia (USP) resolution from retention and peak shape data. Figure 4 shows a screenshot illustrating some of the new functionalities of PeakTracker within the Fusion QbD software.

Solvents and Gases for HPLC and MS
Leman Instruments introduced gas generators to provide high-purity nitrogen and hydrogen (99.999%) at a flow rate up of 250 or 500 mL/min for GC and MS instruments.
Merck KGaA (MilliporeSigma) introduced a new line of ultrapure LC–MS-grade solvents (LiChrosolv Brand, acetonitrile, methanol, and water) that have lower levels of particulate and chemical contaminants, including lower levels of polyethylene glycol (PEG).
Other Separation Systems
Postnova introduced the EAF2000 Electrical Flow FFF series, a separation system based on the principle of electrical and asymmetrical field-flow fractionation (FFF) for the separation of particles, polymers, and proteins. Separation by particle size and particle change based on electrophoretic mobility can be achieved. Shimadzu Scientific Instruments introduced a new Prep SFC system in collaboration with the Emerging Technologies Consortium to support purification in drug discovery and other industries.
Summary
This instalment summarizes new HPLC and MS products introduced at Pittcon 2019 and in the prior year and describes modern trends of these products and the market for them.
Acknowledgements
The author thanks the marketing staff of all manufacturers who provided timely responses to the LCGC questionnaires. The author is grateful to Glenn Cudiamat of TopâDown Analytics, Shawn Anderson from Agilent Technologies, Brian Murphy, Tom Walter, and Isabelle VuTrieu of Waters, Alice Krumenaker of TW Metals, Yingchun (Jasmine) Lu of Shimadzu, Peter Zipfell and Yan Chen of Thermo Fisher Scientific, Jihui Li of Brightside Scientific, and Humberto Rojo of Hycor Biomedical, for providing useful inputs and comments.
The content of this article are the opinions of the author from gathered data from open literature, websites, personal networking, and observations at Pittcon 2019, and bears no relationship to those of LCGC, Pittcon, or any other organization.
References
- C.H. Arnaud, Chem. & Eng. News94(24), 29–35 (2016).
- “Chromatography Instruments Market Worth 10.99 Billion USD by 2022”, Markets and Markets, press release. http://www.marketsandmarkets.com/PressReleases/ chromatography-instrumentation.asp, accessed 15 March 2019.
- “Global Mass Spectrometry Market Size, Market Share, Application Analysis, Regional Outlook, Growth Trends, Key Players, Competitive Strategies and Forecasts, 2015 to 2025”, press release, Research and Markets. https://www.researchandmarkets.com/reports/4313373/global-mass-spectrometry-market-size-market, accessed 15 March 2019.
- R.L. Wixom and C.L. Gehrke, Eds., Chromatography: A Science of Discovery (Wiley, Hoboken, New Jersey, USA, 2010).
- D. Guillarme and M.W. Dong, Eds., Trends Anal. Chem.63, 1–188 (2014) (Special issue).
- M.W. Dong, LCGC Europe30(6), 306–313 (2017).
- M.W. Dong, HPLC and UHPLC for Practicing Scientists, 2nd Ed. (Wiley, Hoboken, New Jersey, USA, 2019), Chapter 4, in press.
- Agilent Biocolumns, Amino Acid Analysis, “How-To” Guide, 5991-7694EN, Agilent Technologies, March 2018.
- Routine Peptide Mapping Analysis Using the BioAccord System, Waters BioAccord Technology Brief, 7200006466 EN, Waters Corporation, Milford, Massachusetts, USA, 2019.
- Fully Automated Sample Preparation Module for LCMS: CLAM-2030, C297-E124A, Shimadzu Corporation, 2018.
Michael W. Dong is a principal of MWD Consulting, which provides training and consulting services in HPLC and UHPLC, method improvements, pharmaceutical analysis, and drug quality. He was formerly a Senior Scientist at Genentech, a Research Fellow at Purdue Pharma, and a Senior Staff Scientist at Applied Biosystems/PerkinElmer. He holds a Ph.D. in analytical chemistry from City University of New York, USA. He has more than 100 publications and a best-selling book in chromatography. He is an editorial advisory board member of LCGC North America and the Chinese American Chromatography Association. Direct correspondence to: LCGCedit@mmhgroup.com

















New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
2 Commerce Drive
Cranbury, NJ 08512

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)