Automated Solid Phase Extraction of Allantoin from Cosmetics and Topical Pharmaceuticals Prior to Analysis by HPLC
Allantoin is a heterocyclic organic compound derived from purine.
Michael Halvorson1, Torsten Kretschmer2, and Martin Roedel2, 1Gilson, Inc., and 2Macherey-Nagel GmbH
Allantoin is a heterocyclic organic compound derived from purine. Allantoin has a long history of use in a variety of topical pharmaceuticals and cosmetics for skin care due to its keratolytic, moisturizing, soothing, and anti-irritant properties. Allantoin is typically used in these products at a level of 0.1 to 2.0%. Solid phase extraction (SPE) is often used as a purification step prior to analysis of allantoin in these products due to the complex nature of the matrices.
This study describes an automated SPE protocol using a Gilson GX-271 ASPEC™ System for the extraction of allantoin from cosmetics prior to analysis by HPLC using a NUCLEODUR® 100-3 HILIC column.
Experimental Conditions
Mix 1 g of sample containing allantoin with 100 mL of ultra-pure water. Allantoin-free cosmetics/topicals were spiked with 5 mg allantoin.
Solid Phase Extraction (SPE) Protocol
The SPE procedure used 3 mL Macherey-Nagel CHROMA-BOND®HR-XA (60 mg) cartridges.The SPE protocol is entirely automated using the Gilson GX-271 ASPEC system. The SPE steps are summarized with the schematic provided in the GX-271 ASPEC control software, Trilution LH™ (Figure 1).
- Initialization Step: Gilson Mobile SPE Racks are moved above the waste rack
- Condition the cartridge with 1 mL of methanol at 0.5 mL/min
- Condition the cartridge with 1 mL of ammonia, w(NH3) = 5% at 0.5 mL/min
- Dispense 4 mL of sample( 1g in 100 mL water) into a tube at 5 mL/min
- Dispense 400 µL ammonia, w(NH3) = 26% at 0.5 mL/min into the same tube as step above
- Load 1.1 mL of the sample mix created above onto the SPE cartridge at 0.5 mL/min
- Wash cartridge with 1 mL of ammonia, w(NH3) = 5% at 0.5 mL/min
- Wash cartridge with 1 mL of methanol at 0.5 mL/min
- Dry with 5 mL air, 3 mL/min
- Move the Gilson Mobile SPE Rack over the collection tubes
- Elute with 2× 600 µL Hydrochloric acid, HCl, 0.1 mol/L at 0.5 mL/min
- Eluent can be injected directly into the HPLC system
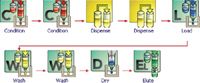
Figure 1: Trilution LH SPE and liquid handling tasks for extraction of Allantoin.
HPLC Analysis
Column: Macherey-Nagel EC 125/3 NUCLEODUR® 100-3 HILIC (Part no. 760 531.30)
Conditions:
Eluent A: 10 mmol/L Ammonium chloride, pH 3.0 20
Eluent B: Acetonitrile 80
Flow Rate: 0.3 mL/min
Temperature: Ambient
Injection Volume: 20 µL
Concentration: β(Allantoin) = 5 µg/mL eluent
Detection: UV, 214 nm
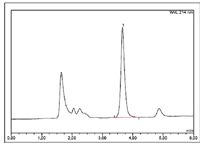
Figure 2: Chromatogram of Allantoin from cosmetic product following SPE extraction. The retention time for Allantoin is 3.66 min.
Results
The recovery of allantoin from the cosmetic product was 85.5% (n = 3).
CHROMABOND and NUCLEODUR are registered trademarks of Macherey-Nagel GmbH. ASPEC and Trilution are trademarks of Gilson, Inc.
Gilson, Inc.
3000 Paramenter St., P.O. Box 620027, Middleton, WI 53562
tel. (608) 836-1551, fax (608) 831-4451
Website: www.gilson.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














