Whither Gas Chromatography? New Tools ~ New Solutions
We might well ask “Where is gas chromatography (GC) heading?” For many analysts, the answer may be just “more of the same,” reflecting that GC is mature and that most analysis tasks and sample types have been tried and tested. In this scenario, any changes to the basic method may be marginal—sample introduction, and maybe a new detector? But beneath this status quo is an undercurrent of passion, excitement, and power.
At the risk of sounding like a broken record, it behooves us to continue the theme of the topic we mentioned in our 35th anniversary issue, which is showcasing the advantages of multi-dimensional separations. For the 40th anniversary issue of LCGC North America, we focus primarily on comprehensive two-dimensional gas chromatography (GC×GC). There is no better way to celebrate this respected magazine, which has charted and chronicled many of the developments in chromatographic separations over its 40-year history, than to reflect on the history of GC×GC since its first demonstration in 1991. Although the first 10 years saw GC×GC struggling to justify its position for ultrahigh resolutions and lacking mainstream appeal, it is evident in 2022 that the situation has changed significantly.
Applications of GC×GC are as broad and extensive as for GC, testifying to the interest in evaluating the technique against one-dimensional (1D) methods, especially for complex mixtures and matrices. There are now legions of reports extolling the virtues of GC×GC, but often these reports also discuss the limitations and challenges that may accompany the technique. It is apparent that an increasing number of researchers are attracted to the novel capabilities inherent in having a high resolution two-dimensional (2D) display of volatile analytes to achieve precise and expansive sample profiling, a greater knowledge of sample composition, and facile sample-to-sample comparison. However, the use of advanced mass spectrometry (MS) approaches, such as the now numerous tandem MS (MS/ MS) tools for GC analysis, is still not well investigated. In many cases, these approaches are not applicable to the speed of modulated peaks in GC×GC. Because MS/MS is often praised and utilized for its analysis selectivity for samples that are not well resolved, the high resolution of GC×GC makes the use of MS/MS less relevant. Additional clarity arises from the use of MS, especially when underlying matrix and phase bleed are effectively removed by the selectivity of the two-column operation in GC×GC.
Over the last 10 years or so, what new processing, operations, and applications have emerged to manipulate and decorate the technique? The basic method involves decisions on modulator type, column set selection (relative dimensions of the columns and their phases), operating conditions, software options, the degree of data interpretation required, and the use of advanced data processing solutions. Innovations include adjustable selectivity, integrating multi-dimensional approaches with GC×GC to improve separation, and chemometric data processing that serves to rationalize trends and features from the complex 2D data sets. However, there is still reluctance in the analytical world to embrace the power of this technique. Perhaps the fact that 1D-GC, and especially 1D-GC–MS, has served the GC community so well over many years, could mean that changing to a new technique—which understandably requires a considerable mindset adjustment—faces some inertia. The new technical requirements, the lack of standard methods, the lack of trained analysts (and managers!), the need for further education, and so on, may explain the reluctance of many instrument companies, industrial analysts, and academic users of GC to venture into this sunrise technology. Yes, effort is required to master GC×GC, but isn’t superior chromatography the culmination of all our analytical dreams?
Among the important applications that now support the demonstration of enhanced separations, we wish to highlight recent developments in analyzing biological samples. Metabolomics should be where GC×GC excels, simply because it is—or should be—the ultimate in untargeted profiling of volatile compounds in samples. A number of years ago, we applied GC×GC–time-of-flight (TOF)-MS to inborn errors of metabolism (IEM) (1). Having a control sample—here, a urine sample was used where IEM was absent—does allow contrast with a positive sample where the IEM is suspected. However, it can still be difficult to identify specific compounds of interest. Usually, it is a case of upregulated compounds showing increased peak abundance. Here, a previously unreported compound was sufficiently resolved to recognize its increased abundance and to allow identification of a compound that could be related to aciduria, which offered a new IEM target that could be added to the screening protocol. Given the usually large matrix present in urine, that strongly overlaps IEM in a 1D-GC screen, the likelihood of identifying such a compound with 1D-GC–MS will be remote. On the MS side, the routine use of MS/MS technology is essentially a targeted method, but choosing the precursor and product ions for unknown compounds is understandably uncertain. As an alternative to GC×GC to help overcome this limitation, a study could utilize high-resolution MS (HRMS) or screen a large cohort of positive and control samples, and then apply chemometrics methods to identify potential analytes as biomarkers.
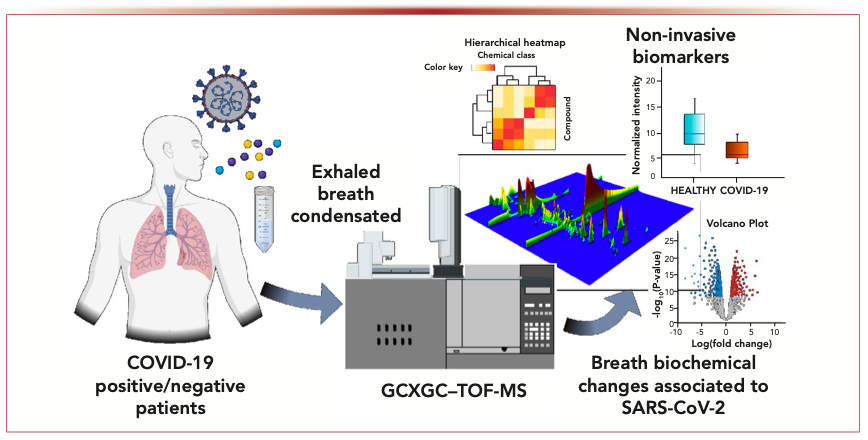
As an approach to investigate cancers, Covid-19, and other disease states, breath analysis has been an emerging active field, although the studies are still few in terms of the populations surveyed, typically ranging from 10 to 100 subjects. Exhaled human breath analysis in active pulmonary tuberculosis diagnostics is an example of such a study, using GC×GC–TOF-MS with chemometrics data interpretation (2). Figure 1 offers an overview of the workflow in such a study. The discovery of biomarkers responsible for or indicative of the disease is significantly enhanced by GC×GC technology, with estimates ranging from 2–10 times greater yield during comprehensive profiling (3). However, the field is constrained by three main issues. First, there is no breath chemical standard mix. Second, instrument software is unable to adequately process untargeted data, meaning that researchers have to use expensive commercial tools or build their own data pipelines. Third, most compounds that are considered biomarkers are not especially “novel,” and they are not unique to a regular breath screen, but they are enhanced in their response as a result of the disease. In this manner, tuberculosis, cystic fibrosis, inflammatory diseases, and lung cancer metabolomic profiles through breath analysis have been reported (4–7).
FIGURE 1: Experimental design of the study: Exhaled breath condensate molecules were analyzed using untargeted metabolomics. Statistical analysis performed on quantified molecules was used to identify potential biomarkers and breath-biochemical changes associated with SARS-CoV-2 infection. With permission of the author, from Metabolites 11, 847 (2021).
In addition, GC×GC–TOF-MS also plays a role in the profiling of other fluids (8). For example, plasma samples have been analyzed for evidence of acute febrile illnesses (9). In urine, studies have sought biomarkers for bladder cancer and isolated urinary steroids contributing to endocrine disorders (10). The headspace of microorganisms has also been investigated, leading to a greater understanding of their metabolic processes and phenotypes. Thus, the “volatilome” comprises unique information of, for example, Pseudomonas aeruginosa, a bacterium that can cause chronic lung infections in individuals with cystic fibrosis (11).
Notably, a fully automated portable 2D-GC–micro-photoionization detector system that has the capabilities to perform GC×GC operations (via rapid contiguous heart cuts over the 1D elution) has been recently reported for the on-site clinical monitoring of exhaled human breath to differentiate acute respiratory distress syndrome (ARDS) from non-ARDS (12). Interestingly, this study demonstrated the gradual acceptance and transition of GC×GC technologies from benchtop to close-to-real-time clinical applications (13). We cannot explore each one of these here, save to say that the relevant studies universally support the value of the discovery and discriminatory steps that GC×GC provides.
Perhaps the most pertinent comment here is that it is impossible to walk away from or ignore the incredible information content of GC×GC. Advances made across the board in many fields of research, from petrochemicals to pesticides, with the extra informing power of accurate mass MS, and to food contaminants and flavors, all testify to the reality of this new technique in our toolbox. The additional costs in hardware, software, training, and validating methods associated with GC×GC may act as an anchor against progress that a full wind in the sails might expect. However, as we move toward a turnkey system, the future of GC×GC remains bright and robust biomarker and profiling research applications are anticipated.
References
(1) K.A. Kouremenos, J. Pitt, and P.J. Marriott, J. Chromatogr. A 1217, 104–111 (2010).
(2) Ref X.M. Beccaria, C. Bobak, B. Maitshotlo, T.R. Mellors, G. Purcaro, F.A. Franchina, C.A. Rees, M. Nasir, A. Black, and J.E. Hill, J. Breath Res. 13, 016005 (2019).
(3) C.L. Jenkins and H.D. Bean, J. Breath Res. 14, 016007 (2020).
(4) C.A. Bobak, L. Kang, L. Workman, L. Bateman, M.S. Khan, M. Prins, et al, Sci. Rep. 11, 1–9 (2021).
(5) M.A. Iqbal, Chemical profiling of exhaled breath from cystic fibrosis subjects using comprehensive two-dimensional gas Chromatography, UTS Digital Thesis Collection. https://opus.lib.uts.edu.au/handle/10453/148951 (accessed 2021).
(6) N.D. Giovanni, M.-A. Meuwis, E. Louis, and J.-F. Focant, J. Proteome Res. 19, 1013–1028 (2020).
(7) H. Ma, X. Li, J. Chen, H. Wang, T. Cheng, K. Chen, and S. Xu, Anal. Methods 17, 6841–6849 (2014).
(8) Y.F. Wong, C. Hartmann, and P.J. Marriott, Bioanalysis 6, 2461–2479 (2014).
(9) E. Näsström, C.M. Parry, N.T.V. Thieu, R.R. Maude, H.K. de Jong, M. Fukushima, et al, Elife 6, 1–10 (2017).
(10) K.K. Pasikanti, K. Esuvaranathan, Y. Hong, P.C. Ho, R. Mahendran, L. Raman Nee Mani, et al, J. Proteome Res. 12, 3865–3873 (2013).
(11) T.J. Davis, A.V. Karanjia, C.N. Bhebhe, S.B. West, M. Richardson, and H.D. Bean, Sphere 5, e00843-20 (2020).
(12) M. Zhou, et al, Anal. Bioanal. Chem. 411, 6435–6447 (2019).
(13) J.J. Whiting, E. Myers, R.P. Manginell, M.W. Moorman, J. Anderson, C.S. Fix, et al, Lab. Chip 19, 1633–1643 (2019).
Philip J. Marriott is with the Australian Centre for Research on Separation Science in the School of Chemistry at Monash University, in Clayton, Australia. Direct correspondence to: philip.marriott@monash.edu

Yong Foo Wong is with the Centre for Research on Multidimensional Separation Science, in the School of Chemical Sciences at the Universiti Sains Malaysia, in Penang, Malaysia.

Jane E. Hill is with the Department of Chemical and Biological Engineering at the University of British Colombia, in Vancouver, Canada.


New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.