Development of Highly Sensitive LC–MS and CE–MS Methods for In-Depth Proteomic and Glycomic Profiling of Limited Biological Samples
Informative and deep proteomic and glycomic characterization of limited availability biological and medical samples has been a significant challenge. Here, we describe our current and recent efforts in advancing sample preparation as well as miniaturized electric field- and pressure-driven separation approaches interfaced with high-end mass spectrometry (MS) to enhance the sensitivity and depth of proteomic and glycomic profiling of several types of limited biological and clinically relevant samples.
Alexander Ivanov of Northeastern University

Mass spectrometry (MS) is one of the most extensively used, sensitive, and powerful techniques to study and characterize metabolomes, lipidomes, proteomes, and glycomes of complex biological systems. Despite many recent advancements, the sensitivity of proteomic and glycomic analyses is still limited, especially for scarce amounts of biological samples (for example, microsampled liquid biopsies, microneedle biopsies, populations of rare cells, and even single cells). Many factors impact the sensitivity, including material losses during sample preparation, high MS background noise, MS signal interference because of contaminants (for example, traces of chaotropic agents used in cell lysis or side products of labeling procedures), ionization suppression because of the presence of salts, suboptimal ion transfer into the MS instrument, and inefficient chromatographic or electrophoretic separation. In this article, we outline a few of our most recent research efforts and activities aimed at improving the sensitivity of molecular profiling.
Materials and Methods
The HeLa protein digest standard was obtained from Pierce (Thermo Fisher Scientific) and was resuspended in 1% formic acid (FA) in water. In the analysis of the HeLa digest, an in-house polymerized poly(styrene-co-divinylbenzene) (PS-DVB) monolithic, 35 cm long, 20 μm internal diameter (i.d.) was coupled to an Orbitrap Fusion Lumos MS instrument (Thermo Fisher Scientific) and the acquired data were searched using Proteome Discoverer (version 2.5, searched with SequestHT, ≤1% false discovery rate [FDR]). Extracellular vesicles (EVs) were isolated using a biphasic multimode column in-house packed with CaptoCore 400 and Sepharose CL-2B beads (Cytiva), that we named a sandwich 400 (SW 400) column. The resulting peptides were separated on a 15 cm long, 75 μm i.d., C18 bead-packed reversed-phase (RP) column (1.9 μm ReproSil-Pur, 120 Å C18-AQ beads, Dr. Maisch, Ammerbuch, Germany) on an Ultimate 3000 nano-liquid chromatography (LC) system coupled to Thermo Fisher Scientific’s Orbitrap Exploris 480 MS instrument. All capillary electrophoresis–MS (CE–MS) experiments were carried out with bare fused silica OptiMS capillaries (Sciex, 30 μm i.d. x 150 μm outer diameter x 90 cm) coupled to various MS instruments via a sheathless interface (CESI 8000, Sciex). In CE–MS-based bottom-up proteomic profiling experiments, Thermo Fisher Scientific’s Orbitrap Fusion Lumos was used in positive electrospray ionization (ESI) mode. Capillary zone electrophoresis–MS (CZE-MS) and capillary zone electrophoresis–tandem MS (CZE-MS2) data were processed with GlycReSoft (v. 3.10) software (Boston University) and SimGlycan (v. 5.91) software (Premier Biosoft), respectively. CZE–MS methods to profile N-glycans were conducted using a Q Exactive Plus Orbitrap MS (Thermo Fisher Scientific) instrument in negative ESI mode. Proteome Discoverer (version 2.5), searched with the SequestHT algorithm with Inferys rescoring (1) and filtered at a ≤1% FDR, was used to search the EV-related proteomic data (bead-packed column) and in CE–MS analysis of a 10-cell equivalent.
Results
Sample preparation is one of the most critical steps to achieve high sensitivity. Proteomic bottom-up or top-down sample preparation workflows are typically done in-solution in an Eppendorf tube with a relatively large total volume. Such sample processing techniques are appropriate for larger-scale sample amounts (μg or mg amounts of protein), but they are not suitable for limited complex samples (pg or μg amounts of protein) because of significant nonspecific protein and peptide losses caused by adsorption on various surfaces. To overcome this challenge, our group has developed a novel bottom-up workflow that we named On-microSolid-phase Extraction Tip (OmSET) sample preparation for limited biological samples (2). This miniaturized method builds upon the stop-and-go extraction tip (StageTip) approach (3) and the findings in sample processing previously described by our group (4). The micro-solid-phase extraction (micro-SPE) tips consist of several C18 particle-loaded porous microfiber membrane discs inserted inside a 10 μL pipette tip, where all stages of the sample preparation workflow occur, from cell lysis to protein digestion. This approach allows for minimal sample processing volumes (1−3 μL) and a broad range of protein amounts (tested range 200–10,000 U937 cells roughly corresponding to 70–3,500 ng of protein) (5) to be processed with minimal sample losses since the number of steps (including the desalting cleanup and sample transfer steps) is dramatically reduced. Similar to the OmSET method, we developed another miniaturized in-solution technique to process limited samples (6). This workflow allows all sample processing steps—starting from sample deposition to final sample injection—to be performed in a glass sample insert that can be placed directly into instrument-specific autosampler vials. This protocol further minimizes sample losses by eliminating one sample pipetting step (that is, transfer of the digested sample to the sample injection vial). Therefore, the processed sample can be injected for CE or nanoflow liquid chromatography (nLC) analysis directly from the vial where it was prepared.
To increase the sensitivity in the analysis of highly heterogeneous limited samples and to facilitate data interpretation, nanoflow liquid separation techniques are often coupled to MS. CE and nLC are nanoscale separation techniques, typically used in MS-based proteomic and glycomic profiling of limited samples, and both techniques are extensively used in our laboratory (Figure 1). In CE and nLC, the analytes are injected in fused silica capillary columns of various lengths and internal diameters. Currently, several nLC capillary bead-packed column formats are commercially available, with typical column internal diameters ranging from 50 to 100 μm. However, further miniaturized capillary columns with internal diameters less than 50 μm may play a crucial role in applications where high sensitivity is required and where the sample amount is limited (for example, single cells and small cell populations). Such nLC columns are challenging to pack, and they are currently not commercially available. Therefore, their production and use are mostly limited to academic laboratories. Columns with a narrow i.d. provide enhanced sensitivity because of the reduced radial dilution of chromatographic bands (7). Moreover, these columns are typically operated at an ultra-low flow (ULF, flow rate ≤25 nL/min), and their coupling to MS results in a further increase in the sensitivity because of enhanced ESI efficiency, improved transfer of ions into the MS, and reduced ionization suppression. Similar results can be obtained in CE–MS analysis with a sheathless interface using 30 μm i.d. capillaries and the separation conducted with ULF (8).
Figure 1: An overview of the capillary-based separation formats being developed and used in the Ivanov Laboratory. (a) Schematics of the fused silica capillary tubing protected by a polyimide coating. Schematics and typical dimensions from a top view of (b) capillary electrophoresis, (c) nLC—bead-packed, (d) nLC—porous layer open tubular, and (e) nLC—monolithic separation columns. Gray structures inside the capillaries represent the stationary phase used for LC separations. Schematic images and capillary dimensions are not in scale.
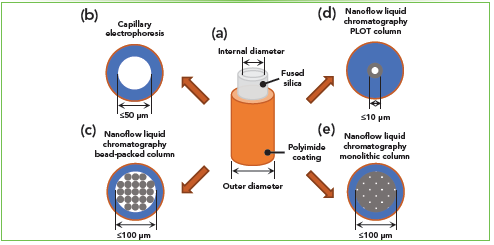
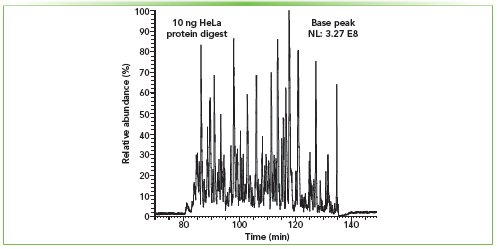
In our laboratory, there are three main types of nLC columns in a capillary format that have been used for the ultrasensitive analysis of limited biological samples. The first type is a bead-packed column (Figure 1c) used to evaluate the OmSET workflow (2) and in the analysis of EVs. There are multiple options for commercially available and in-house packed nLC columns with various bead types (porous, non-porous, and superficially porous), sizes, and chemistries. The second column type that we use in our research and continuously advancing is the open tubular (OT) or porous layer open tubular (PLOT) capillary columns (Figure 1d), in which a thin layer of a stationary phase is attached to the interior surface of the capillary. PLOT columns are versatile and can be used in a variety of applications—from the analysis of small molecules to the analysis of peptides and intact proteins (9). We are specifically interested in advancing the PLOT column technology to enhance the sensitivity of MS-based top-down and bottom-up proteomic profiling of small-cell populations. The third type of nLC capillary column we are actively developing and using in our laboratory is the monolithic column (Figure 1e). The separation medium of a monolithic column bed consists of a continuous fully porous rod-like material that is covalently attached to the capillary inner wall. The polymeric monolithic stationary phase that we have been using lately is synthesized by temperature-induced radical polymerization of monomers (for example, styrene and 1-octadecene (C18)) and a crosslinker (for example, divinylbenzene) inside the capillary. A monolithic column prepared this way can be used either as a precolumn or as an analytical column (4,10). Figure 2 shows the separation of the 10 ng HeLa protein digest standard (corresponding to approximately 25 average-size cells) using a 35 cm long and 20 μm i.d. monolithic nLC column (10). This type of column is prepared by copolymerization of styrene, divinylbenzene, and 1-octadecene. The C18 moieties are added to the polymerization mixture to increase the column hydrophobicity. We typically operate such ULF monolithic columns at a flow rate of ~12 nL/min. The follow-up database searches after the LC–MS analysis resulted in, on average, 3,244 ± 24 protein group and 14,015 ± 118 peptide group identifications (filtered at ≤1% FDR) (10). The gains in profiling depth typically correspond to ~20–30% over a 75 μm i.d. bead-packed column. However, even presently, there are still many challenges that hamper a broader use of ULF nLC columns in proteomic studies of limited samples and other applications requiring high sensitivity MS-based detection. For example, specialized nanoflow ultrahigh-pressure liquid chromatography (nUHPLC) pumps, which are capable of reproducible gradient delivery at 1–20 nL/min without splitting the flow, are still unavailable. Also, the separation performance of ULF nLC columns is highly susceptible to any imperfections in plumbing, resulting in dead volumes and other extra column effects compared to conventional 75–100 μm i.d. nLC columns.
Figure 2: Proteomic profiling of low-nanogram samples using ultra-low flow monolithic columns. Monolithic column (PS-C18-DVB chemistry, 20 μm i.d., 35 cm long) used for bottom-up proteomic analysis of 10 ng of the HeLa protein digest standard. Peptides were eluted at a flow rate of 12 nL/min from the column with a 1 h linear gradient from 1% B to 15% B, where solvent A is 0.1% FA in water and solvent B consisted of 0.1% FA in acetonitrile. The analytical approach was the same as previously described (10).
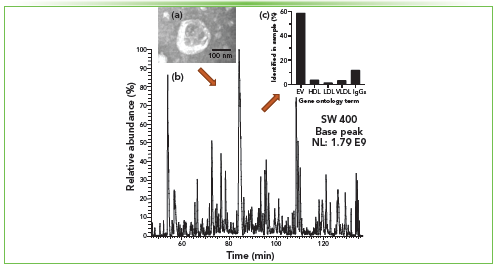
Plasma, serum, whole blood, urine, saliva, and other highly complex physiological fluids can be used as sources of EVs. EVs are nanoscale lipid membrane-enclosed structures that are secreted by various cell types. EVs can be involved in cell–cell communication that may influence diverse biological pathways. EVs demonstrated the potential for applications in early diagnostics of different pathologies and targeted therapeutics. EVs have been characterized by multiple and complementary analytical techniques, including nLC coupled to MS to profile EV-related proteins that have been attributed to EV biogenesis and disease progression (11). However, there is still a lack of standardization in the EV field for sample procurement, isolation, and EV and biomolecule analysis (12). We developed several methods to help alleviate this issue, specifically pertaining to EV-derived protein analysis. Utilizing nLC coupled to MS, lower abundance proteins within EVs can be profiled and screened for biomarkers specific to certain disease states. To do so, the EVs must be isolated from other highly abundant macromolecules present in the bioogical matrices, such as proteins, protein complexes, and apolipoprotein particles. There are various reported ways to isolate EVs from biological fluids, including the gold standard, ultracentrifugation (UC) (12). However, UC-based EV enrichment co-isolates highly abundant apolipoprotein particles because of the similar densities between apolipoproteins and EVs, leading to a contaminated sample (13). To combat this issue, biphasic multimode columns, including size-exclusion chromatography (SEC) Sepharose CL-2B and mixed-mode CaptoCore (400 or 700) stationary phases, were developed in our laboratory. By using this biphasic isolation technique, apolipoproteins, as well as free plasma proteins and protein complexes, can be efficiently depleted while retaining EVs, leading to a higher purity EV isolate. The EVs enriched with a biphasic multimode column from plasma were processed using the OmSET digestion method (2), and the released peptides were loaded onto a nLC C18 bead-packed column (75 μm i.d., 15 cm long) coupled to an Orbitrap Exploris 480 mass spectrometer (Thermo Fisher Scientific). We quantified on average 823 ± 96 proteins (filtered at ≤1% FDR) in EVs isolated from 100 μL of human blood plasma, among which approximately 60% are proteins commonly found in EVs (Figure 3).
Figure 3: Isolation and proteomic profiling of plasma extracellular vesicles. (a) The left top insert shows a representative transmission electron microscopy (TEM) image of an EV. (b) Base peak chromatogram of digested EV proteins from 100 μL human blood plasma isolated from a biphasic multimode column. A 90-min linear gradient from 1% B to 25% B, where solvent A is 0.1% formic acid (FA) in water and solvent B is 0.1% FA in acetonitrile, was used. (c) Results of gene ontology term enrichment analysis.
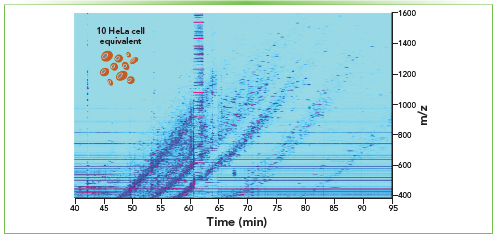
CE (CZE) is a powerful separation technique alternative to LC, with the advantage of high separation efficiency, typically short analysis time, and very low sample volume and background electrolyte (BGE) consumption. In CE (Figure 1b), analytes are separated in an electric field according to their charge and hydrodynamic volume. For some applications, the inner surface of the bare fused capillaries can be treated to alter the physicochemical properties of the surface (for example, charge) and potentially minimize, eliminate, or reverse the direction of the electroosmotic flow. Our group specializes in developing highly sensitive CE–MS methods for proteomic (bottom-up, top-down, and native) and glycomic profiling of mass-limited and complex biological samples. Recently, CE–MS analysis of a 10-cell equivalent injected from approximately 1000 HeLa cells processed by our miniaturized in-solution digestion protocol resulted in the identification of, on average, 1286 ± 5 protein groups and 5,617 ± 119 peptide groups (6). Figure 4 shows an example of an ion density map of this ultra-sensitive CE–MS-based bottom-up proteomic analysis of 10 HeLa cell equivalent injections (6).
Figure 4: CE–MS in bottom-up proteomic profiling of mass-limited samples. Ion density map (intensity level: 5.0 E5) of the CZE–MS analysis of a 10-cell equivalent injected from ~1,000 HeLa cells processed by our miniaturized in-solution digestion protocol. A separation voltage of 20 kV was applied in normal polarity. The experimental techniques were the same as previously described (6).
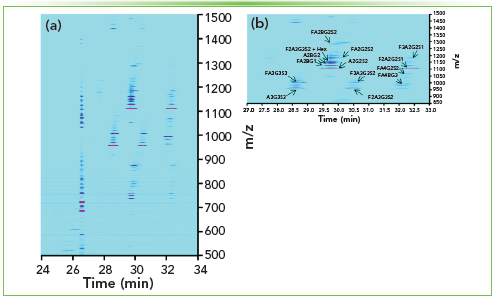
Additionally, CZE–MS methods were developed in our group to profile N-glycans released from minute amounts of blood-derived samples or mammalian cells (14). To achieve the best sensitivity, dopant-enriched nitrogen (DEN) gas, introduced in the nanospray microenvironment, was used in combination with optimized sample preparation (use of carboxyl-coated magnetic microparticles for the enrichment and release of N-glycans) and optimized CZE–MS conditions (that is, optimized CE pressure, ionization, desolvation, and MS parameters). Over 400 and 500 N-glycans could be detected and identified in the EV isolate and whole plasma, respectively, for injected sample amounts equivalent to 340 nL and 14 nL of human plasma, respectively. Figure 5a shows a characteristic ion density map of the CZE–MS analysis of the total EV isolate. Two of the most abundant N-glycans detected in the EV isolate are A2G2S2, a biantennary disialylated glycan, and A3G3S3, a tri-antennary trisialylated glycan. In addition, a large number (approximately 70%) of sialylated glycans (up to five sialic acid residues), among which low abundance ones, were detected in the EV isolate (Figure 5b) (14). The developed and optimized CZE–MS methods were also applied to the profiling of N-glycans released from cancer mammalian cells treated with selected types of cytokines. In our preliminary experiments, over 300 N-glycans were detected and identified in the cell-derived samples using injected amounts equivalent to approximately 50 cells. Different levels of fucosylation (up to seven fucose residues) and sialylation (up to 14 sialic acid residues) were detected in the treated cells.
Figure 5: CE–MS in N-glycan profiling of EVs isolated from blood plasma. (a) Ion density map (intensity level: 2.0 E6) of the CZE–MS analysis of human blood-derived total EV isolate. Panel (b) shows a zoomed-in portion of the same density map at a selected time (min) and m/z range. N-glycans were labeled with 8-aminopyrene-1,3,6-trisulfonic acid (APTS) before their CZE–MS analysis in negative ESI mode. APTS-labeled N-glycans were separated by applying 20 kV in reverse polarity, and the supplemental CZE pressure was switched off for 16 min before applying 5 psi during the rest of the analysis. Sample injections were performed at 5 psi for 40 s, which corresponds to ~34 nL injection volume (that is ~5.3% of the capillary volume) and the amount of EV isolate released from ~340 nL of plasma (that is ~1 μL of human blood). The experimental techniques were the same as previously described (14).
Conclusions
The above-described projects and developed approaches outline only some of our recent and ongoing research efforts in the field of high sensitivity molecular profiling based on the miniaturized separation coupled to MS. Our additional current research directions also include different modes of micro- or nano-scale separations coupled to MS to enable high sensitivity and increased depth of molecular profiling and structural characterization of intact cellular proteomes, biopharmaceutical proteins, new modalities of biotherapeutics, non-covalent protein-protein and protein-drug interactions, and metabolites to solve biological and clinical puzzles and challenges. We believe that miniaturized and ULF liquid phase separations coupled with MS will progress further to enable numerous exciting and long-awaited applications to advance fundamental biology and medicine.
Acknowledgments
We are thankful to all our current and past collaborators. We are grateful for the financial support of the various projects described here provided by the National Institutes of Health under award numbers R35GM136421 (A.R.I) and R01CA218500 (A.R.I). The authors acknowledge Thermo Fisher Scientific for their support through a technology alliance partnership program (A.R.I), Sciex for providing CESI capillaries and support, and the Dana-Farber Cancer Institute/Northeastern University Joint Program in Cancer Drug Development, for additional financial support.
References
(1) S. Gessulat, T. Schmidt, D.P. Zolg, P. Samaras, K. Schnatbaum, J. Zerweck, T. Knaute, et al, Nat. Methods 16(6), 509–518 (2019). DOI: 10.1038/s41592-019-0426-7.
(2) J.C. Kostas, M. Gregus, J. Schejbal, S. Ray, and A.R. Ivanov, J. Proteome Res. 20(3), 1676–1688 (2021). DOI: 10.1021/acs.jproteome.0c00890.
(3) J. Rappsilber, Y. Ishihama, and M. Mann, Anal. Chem. 75(3), 663–670 (2003). DOI: 10.1021/ac026117i.
(4) S. Li, B.D. Plouffe, A.M. Belov, S. Ray, X. Wang, S.K. Murthy, B.L. Karger, and A.R. Ivanov, Mol. Cell Proteomics 14(6), 1672–1683 (2015). DOI: 10.1074/mcp.M114.045724.
(5) R.G. Huffman, A. Chen, H. Specht, and N. Slavov, J. Proteome Res. 18(6), 2493–2500 (2019). DOI: 10.1021/acs.jproteome.9b00039.
(6) K.R. Johnson, M. Gregus, J.C. Kostas, and A.R. Ivanov, Anal. Chem. 94(2), 704–713 (2022). DOI: 10.1021/acs.analchem.1c02929.
(7) S.R. Wilson, T. Vehus, H.S. Berg, and E. Lundanes, Bioanalysis 7(14), 1799–1815 (2015). DOI: 10.4155/bio.15.92.
(8) J.M. Busnel, B. Schoenmaker, R. Ramautar, A. Carrasco-Pancorbo, C. Ratnayake, J.S. Feitelson, et al, Anal. Chem. 82(22), 9476–9483 (2010). DOI: 10.1021/ac102159d.
(9) T. Vehus, H. Roberg-Larsen, J. Waaler, S. Aslaksen, S. Krauss, S.R. Wilson, and E. Lundanes, Sci. Rep. 6, 37507 (2016). DOI: 10.1038/ srep37507.
(10) M. Gregus, J.C. Kostas, S. Ray, S.E. Abbatiello, and A.R. Ivanov, Anal. Chem. 92(21), 14702–14712 (2020). DOI: 10.1021/acs.analchem.0c03262.
(11) C. Thery, K.W. Witwer, E. Aikawa, M.J. Alcaraz, J.D. Anderson, et al, J. Extracell. Vesicles 7(1), 1535750 (2018). DOI: 10.1080/20013078.2018.1535750.
(12) S. Gandham, X. Su, J. Wood, A.L. Nocera, S.C. Alli, L. Milane, et al, Trends Biotechnol. 38(10), 1066–1098 (2020). DOI: 10.1016/j.tibtech.2020.05.012.
(13) N. Karimi, A. Cvjetkovic, S.C. Jang, R. Crescitelli, M.A. Hosseinpour Feizi, R. Nieuwland, et al, Cell. Mol. Life Sci. 75(15), 2873– 2886 (2018). DOI: 10.1007/s00018-018-2773-4.
(14) A.L. Marie, R. Ray, S. Lu, J. Jones, I. Ghiran, and A.R. Ivanov, Anal. Chem. 93(4), 1991–2002 (2021). DOI: 10.1021/acs.analchem.0c03102.
About the Authors
Michal Gregus, Alan Zimmerman, Anne-Lise Marie, Kendall R. Johnson, and Alexander R. Ivanov are with the Barnett Institute of Chemical and Biological Analysis in the Department of Chemistry and Chemical Biology, at Northeastern University, in Boston, Massachusetts. Direct correspondence to: a.ivanov@northeastern.edu

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.