Let’s Get Small: Miniaturizing Separations for Single-Cell Analysis
Direct profiling of biochemical expression within single cells provides insights into cellular processes that are lost when ensemble averages are measured across populations of cells. Advanced separations coupled with mass spectrometry (MS) can now quantify more than 1000 proteins within single cells. Further miniaturization of separations will greatly extend the reach of single-cell proteomics, metabolomics, and lipidomics, but key challenges in instrumentation, column technology, and ionization sources must be addressed.
It is a remarkable characteristic of electrospray ionization–mass spectrometry (ESI-MS) that analyzing less sample can provide more signal, yet this is easily observed when reducing the flow rate in a direct infusion experiment. Even as the flux of analytes exiting the electrospray emitter decreases, the MS signal may increase because of ionization efficiency gains that more than offset the reduced ion flux (1), which is because of the formation of smaller, more readily desolvated droplets at lower flow rates. Additional benefits of low-flow ESI-MS include reduced ionization suppression and charge competition, providing improved quantitative performance (2).
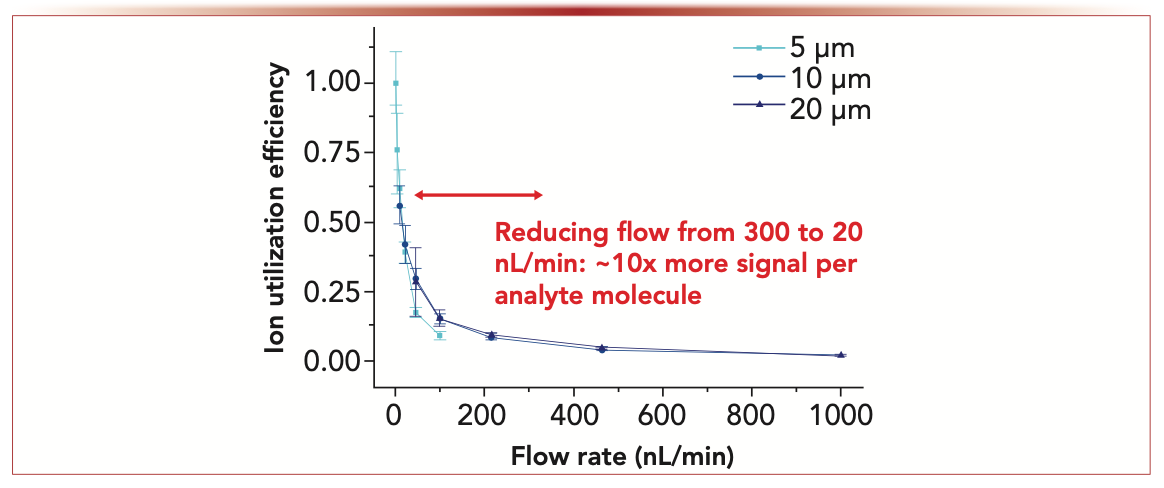
When analyzing the biochemical content of single cells containing mere picograms of analytes, as well as other trace samples such as tissue sections and fine needle aspiration biopsies, it is critical to leverage the sensitivity gains associated with low-flow ESI. With few exceptions (3), it requires operating the liquid chromatography (LC) or capillary electrophoresis (CE) separations that feed the electrospray source at correspondingly low flow rates. How low is low enough? Standard LC flow rates for proteomics applications use 75 μm i.d. capillary columns operating at ~300 nL/min. Although this flow rate is already much lower than that of typical analytical LC columns, it is far from ideal for single-cell applications. For example, reducing the flow rate from 300 nL/min to 20 nL/min increases the amount of MS signal per analyte molecule by a factor of ~10 (Figure 1). We have found in previous studies that ion utilization efficiency and MS signal-to-noise (S/N) ratios continue to increase as flow rates are further reduced to 1 nL/min (4).
FIGURE 1: Relative ion utilization efficiency as a function of flow rate for a standard peptide mixture as measured by direct infusion using chemically nanoelectrospray emitters having different inner diameters.
Given the growing interest in single-cell analysis and the clear benefits of ultralow-flow LC- and CE-MS for such applications, what is standing in the way of routine implementation? There are key challenges pertaining to column technology, instrumentation, and ionization sources that must be overcome, but each of these issues can be addressed. Here, I discuss these challenges and potential paths forward as they relate to LC.
Packed capillary columns having inner diameters of 75 μm and C18 particles in the 1.6–3 μm range have been the industry standard for proteomics applications for well over a decade and are available from a variety of vendors. Operating these columns at flow rates that are much lower than their intended 200–500 nL/min results in reduced separation efficiency. As such, the LC column diameter must be reduced to maintain LC separations at their optimum linear velocity. Unfortunately, packing chromatographic media into smaller diameter capillaries (for example, 20–50 μm) is quite challenging, with particles tending to block the column inlet and a propensity for nonuniform, packed beds. Commercialization efforts could conceivably make such narrowbore separations more broadly available, but these packed columns are rarely demonstrated below ~20 nL/min (5,6). Therefore, this flow rate is still not as low as desired to achieve the absolute lowest detection limits. As a result, a promising direction for further miniaturization of LC is open tubular (OT) and porous layer open tubular (PLOT) chromatography (7,8), as these separations can achieve high efficiencies at very low flow rates and do not require difficult and tedious column packing. The sample loading capacities of OT and PLOT columns is low relative to packed-bed columns, but a large loading capacity is not needed for single-cell analyses, so these columns might be well matched to such applications.
Even with a column that operates ideally at ultralow flow rates, commercial nanoLC instrumentation is poorly suited for these columns. There are extracolumn volumes that must be cleared to transport the sample and mobile phase from their source to the head of the column. These volumes are generally fixed, and the resulting delay times can become insupportably long at ultralow flow rates. For example, the direct loading of the sample from a 1 μL sample loop at 20 nL/min would require 50 min plus the time it takes to clear the connecting tubing, which can easily leave a ~$1M mass spectrometer sitting idle more than half the time. As mentioned above, further miniaturizing LC with OT columns operating at approximately 1 nL/min has proven highly promising for single-cell proteomics (SCP) (8). However, without a suitable injection method, there is currently no way to inject the sample onto these columns without counterproductively wasting a majority of the precious sample. Thus, suitable instrumentation needs to be developed to accommodate much lower-flow LC separations. One potential path forward would be to implement multi-column separations that allow sample to be eluted from one column while the overhead steps of column washing and regeneration, sample loading, and traversing delay volumes take place on the other columns (9,10). For example, if a single nanoLC column is found to actively elute analytes 25% of the time, then a four-column system may be constructed to increase duty cycle to ~100%. Implementing multi-column separations is an active area of research that should receive further attention and investment.
It is also critical for ESI-MS-based analyses that the electrospray emitter provides stable operation. The constraints on emitter geometry become increasingly stringent at ultralow flow rates, where a narrow, flat orifice with very thin walls is required. These emitter geometries have been achieved by means of chemical etching (4,11), but new commercial offerings may also prove capable of such low flows. We have successfully coupled etched emitters to packed columns operating at 20 nL/min with negligible band broadening using zero-dead-volume unions (5,6), but this requires making flawless connections and polishing both the column outlet and the emitter inlet. Thus, it would be highly desirable to have emitters integrated into columns that are designed to operate at these low flow rates.
The challenges in column technology, instrumentation, and ionization must be solved together to have an impact on the emerging field of single-cell ‘omics (12). For example, developing ideal columns and instruments without a means of ionizing the separated analytes will be of no benefit. However, given the rapid development in each of these areas, it is likely that these miniaturized separations will be routinely implemented for single-cell analyses within the next few years, providing insights into biological systems with single-cell resolution that would be otherwise unattainable.
Acknowledgment
The author would like to thank the National Institutes of Health (NIH) and National Institute of General Medical Science (NIGMS) for supporting this work through award R01 GM138931.
References
(1) A. Schmidt, M. Karas, and T. Dulcks, J. Am. Soc. Mass Spectrom. 14(5), 492–500 (2003).
(2) R.B. Cole, J. Mass Spectrom. 35(7), 763–772 (2000).
(3) R.T. Kelly, J.S. Page, R. Zhao, W.J. Qian, H.M. Mottaz, K.Q. Tang, and R.D. Smith, Anal. Chem. 80(1), 143–149 (2008).
(4) I. Marginean, K.Q. Tang, R.D. Smith, and R.T. Kelly, J. Am. Soc. Mass Spectrom. 25(1), 30–36 (2014).
(5) Y. Cong, Y. Liang, K. Motamedchaboki, R. Huguet, T. Truong, R. Zhao, et al, Anal Chem. 92(3), 2665–2671 (2020).
(6) Y. Cong, K. Motamedchaboki, S.A. Misal, Y. Liang, A.J. Guise, T. Truong, et al, Chem. Sci. 12(3), 1001–1006 (2021).
(7) S.Y. Li, B.D. Plouffe, A.M. Belov, S. Ray, X.Z. Wang, S.K. Murthy, et al, Mol. Cell Proteomics 14(6), 1672–1683 (2015).
(8) P.L. Xiang, Y. Zhu, Y. Yang, Z.T. Zhao, S.M. Wil- liams, R.J. Moore, et al, Anal. Chem. 92(7), 4711–4715 (2020).
(9) E.A. Livesay, K.Q. Tang, B.K. Taylor, M.A. Buschbach, D.F. Hopkins, B.L. LaMarche, et al, Anal. Chem. 80(1), 294–302 (2008).
(10) K.G.I. Webber, T. Truong, S.M. Johnston, S.E. Zapata, Y. Liang, J.M. Davis, et al, Anal. Chem. 94(15), 6017–6025 (2022).
(11) R.T. Kelly, J.S. Page, Q.Z. Luo, R.J. Moore, D.J. Orton, K.Q. Tang, and R.D. Smith, Anal. Chem. 78(22), 7796–7801 (2006).
(12) R.T. Kelly, Mol. Cell Proteomics 19(11), 1739–1748 (2020).
Ryan T. Kelly is with Brigham Young University in Provo, Utah. Direct correspondence to: ryan.kelly@byu.edu.


Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.