Use of an Efficient LC Method Development Approach in the Analysis of Terfenadine, Fexofenadine and Azaclonol
LCGC Asia Pacific
Using ACQUITY UPLC technology with triple quadrupole MS detection enhances the selectivity, sensitivity and throughput in quantitative bioanalytical studies. Detection limits for these methods are being driven lower and lower as drugs become more potent.
Using ACQUITY UPLC technology with triple quadrupole MS detection enhances the selectivity, sensitivity and throughput in quantitative bioanalytical studies. Detection limits for these methods are being driven lower and lower as drugs become more potent. For this reason, methods with highest sensitivity possible must be developed. Additionally, this must happen under increasingly tight deadlines to shorten the drug development cycle. We have developed a straightforward, streamlined approach to efficient LC method development. This use of this approach is demonstrated in the generation of a sensitive and selective method for terfenadine and two of its metabolites, fexofenadine and azacyclonol. Fexofenadine, in particular, has gained importance as the commonly prescribed anti-allergic Allegra. The structures for all analytes are shown in Figure 1.
Figure 1
Analytical Challenges
Metabolites and their parent compounds can differ dramatically in chemical properties, making it difficult to develop one chromatographic method that is optimal for all analytes. For example, a non-polar basic parent drug can easily undergo biotransformation into basic metabolites as well as polar acidic metabolites. The LC method must then provide adequate sensitivity for low levels of all of these types of compounds, as well as resolution of structurally similar metabolites from the parent drug.
Experimental
A screening approach was used to choose the best starting chromatographic conditions. Four different column chemistries were screened at high and low pH using two organic modifiers. The resulting chromatograms were evaluated for peak shape, retention and selectivity. From these data, the column chemistry, pH and mobile phase modifier were chosen. The chosen screening gradient was later optimized to decrease run time.
LC conditions for screening
LC system: ACQUITY UPLC and Quattro Premier
Columns: ACQUITY UPLC BEH C18 2.1 × 50 mm, 1.7 μm ACQUITY UPLC BEH Shield RP18 2.1 × 50 mm, 1.7 μm ACQUITY UPLC BEH Phenyl 2.1 × 50 mm, 1.7 μm ACQUITY UPLC HSS T3 2.1 × 50 mm, 1.8 μm
Column temp.: 50 °C
Flow-rate: 600 μL/min.
Mobile phase A1: 0.1% HCOOH in H2O (~pH 2.7)
Mobile phase A2: 0.1% NH4OH in H2O (~pH 11)
Mobile phase B1: MeOH
Mobile phase B2: ACN
Gradient: 5–95% B in 2 min, hold for 0.5 min; return to initial conditions
MS conditions
MS system: Waters Quattro Premier
Ionization mode: ESI Positive
Capillary voltage: 3200 V
Cone voltage: 17 V, 31 V and 47 V for azacyclonol, terfenadine and fexofenadine, respectively
Collision energy: 13 eV, 28 eV and 31 eV for azacyclonol, terfenadine and fexofenadine, respectively
MRM channels: terfenadine m/z 472.6–436.6, fexofenadine m/z 502.4–466.3, azacyclonol m/z 268.2–250.2
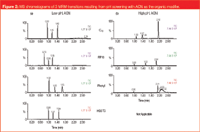
Figure 2
Results and Discussion
Chromatographic Screening: The chromatograms resulting from pH screening with ACN as the organic modifier are shown in Figure 2. Initial optimization was done to determine the MRM transitions and optimal mass spectrometry conditions for each compound. A TIC of the three transitions is shown in the figure. All columns were run at low pH (a). The C18, Shield RP18, and Phenyl columns were also run at high pH (b). (Note: the HSS T3 column is a silica particle and cannot be run at high pH.) Chromatograms were evaluated with respect to peak shape, resolution of metabolites from each other and the parent drug, sensitivity and selectivity. Selectivity shifts were observed for fexofenadine and azacyclonol at high versus low pH, as well as dramatic peak shape differences on the phenyl column for the azacyclonol metabolite. Retention of the basic, non-polar compound terfenadine is dramatically affected by change in pH. Notice that terfenadine is more strongly retained on the C18 column at high pH when it is in its neutral state. A significant increase in MS sensitivity (almost 33) is also observed for this analyte under high pH conditions. Terfenadine now elutes in a higher percentage organic. Droplets which are higher in organic composition are more efficiently desolvated in the MS source, resulting in increased sensitivity. We chose low pH mobile phase because of excellent peak shape for all compounds and improved sensitivity for several compounds. We next screened all column chemistries using two organic modifiers. The chromatograms resulting from organic modifier screening are shown in Figure 3. All columns were run at low pH with MeOH (a) and ACN (b). All three compounds chromatograph as well-resolved, narrow peaks with both MeOH and ACN, with the exception of terfenadine on the phenyl column. However, sensitivity is slightly higher using MeOH as the organic modifier. The final step involved choosing the optimal column chemistry. Examination of the low pH, MeOH chromatograms indicates that the best sensitivity for all compounds is achieved with the HSS T3 column. We optimized the chromatographic method using the HSS T3 column at low pH, with MeOH as the organic modifier.

Figure 3
Conclusions
A straightforward approach to LC method development was successfully employed to determine optimum column chemistry, pH and organic modifier for terfenadine and two of its metabolites. This method will be further optimized to shorten run time and will then become part of an overall bioanalytical method for these compounds. The entire screen consisting of 4 columns, 2 pHs and 2 organic modifiers, took 49 min.
© 2007 Waters Corporation. Waters, The Science of What's Possible, ACQUITY UPLC, and Quattro Premier are trademarks of Waters Corporation. Allegra is a registered trademark of sanofi-aventis US LLC.
Erin Chambers, Christopher Singleton and Diane Diehl, Waters Corp., Milford, Massachusetts, USA.

Waters Corporation
34 Maple Street, Milford, Massachusetts 01757, USA
tel. +1 508 478 2000 fax +1 508 478 1990
Website: www.waters.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











