Column Selection for Two-dimensional LC×LC
LCGC Asia Pacific
Proteins can be separated according to their isoelectric points on an ion-exchange column using pH gradients in the first dimension and according to their hydrophobicity on an RP column in the second dimension.
Multidimensional systems are primarily used to increase the number of separated compounds, n, which is conveniently characterized as the peak capacity, P, which is defined as the maximum possible number of separated compounds with regularly spaced closely adjacent peaks filling the whole available space in the chromatogram. So-called "orthogonal" systems with different retention mechanisms controlling the selectivity of separation show strong multiplication effects on the peak capacity, which in a two-dimensional (2D) system can be theoretically as high as the product of the peak capacities in the first and second dimension: P2D = P1 × P2
A separation system can be regarded as multidimensional when the mechanism of the separation in each dimension is different.1 Early 2D separations mainly used the planar mode, including paper chromatography (PC), thin-layer chromatography (TLC), gel electrophoresis (GE) and combinations of these techniques. Later, 2D GC×GC systems became popular. In a 2D column GC×GC or LC×LC separations, the sample is transferred from the first to the second dimension column either off-line or on-line. In the off-line set-up, the fractions from the first column are isolated, preconcentrated and injected onto the second column. On-line multidimensional systems employ manual or automated sample transfer between two or more columns with uninterrupted flow, via one or more switching valves.
The peak capacity under isocratic conditions in a 1D system can be calculated using Equation 1:2
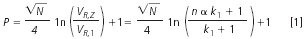
The peak capacity depends primarily on the number of theoretical plates of the column, N, but is strongly affected by the separation selectivity, α, expressed as the relative retention, that is, the ratio of the retention factors of the compounds with adjacent peaks, α = k(i+1) /ki, which is constant over the whole chromatogram with n closely adjacent peaks between the elution volume of the first eluted compound, VR,1, and the elution volume of the last eluted one, VR,Z (with the resolution Rs = 1).
The peak capacity decreases as the retention factor of the first compound k1 increases. However, regular spacing of all peaks over the whole chromatogram required for full use of the peak capacity is rarely achieved in practice and the peak capacity required to separate all sample components at a given probability level increases with the second power of the real number of sample components.3–5
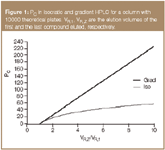
Figure 1
The peak capacity, PG, is generally higher in gradient elution than in isocratic mode within the same range of elution times or volumes (Figure 1), because of approximately constant and significantly narrower bandwidths wg in gradient elution:6
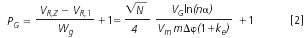
Equation 2 applies in linear gradient reversed-phase liquid chromatography (RPLC), where the effect of the volume fraction of the organic modifier in a binary aqueous–organic mobile phase on the retention under isocratic conditions is — to first approximation — described by Equation 3.7,8

In the derivation of Equation 2, approximately equal retention factors at the time of elution of band maxima, ke, are assumed for all compounds, N is the column plate number, Vm is the column hold-up volume and VG is the volume of the gradient available for the elution of the compounds from the first (elution volume VR,1) to the last one (elution volume VR,Z), corresponding to the change Δφ in the volume fraction of the organic modifier from the start to the end of the gradient.
As is the case for isocratic LC, the gradient peak capacity (PG) controlling the maximum possible number of separated compounds, n, with adjacent peaks strongly depends on the separation selectivity, α, expressed as the ratio of the retention factors of the compounds with n closely adjacent peaks, assumingly constant over the whole chromatogram. The peak capacity increases as the gradient steepness (normalized to the column hold-up volume, VG/(Vm Δφ), and the slope parameter of the Equation 3, m, decrease. The differences in the gradient elution times for samples of homologues or oligomers with different numbers of repeat structural units are approximately constant in gradient elution.
Experimental Set-up
The most common 2D LC×LC technique is fraction heart-cutting, where only the fractions of interest are selected from the effluent of the first-dimension separation system for the separation in the second dimension. In "comprehensive" 2D LC×LC the entire sample is subjected to the two separation mechanisms. The whole effluent from the first dimension is collected in subsequent small-volume fractions transferred into the second-dimension separation system in multiple, repeated alternating cycles by switching a ten-port valve (or two six-port valves) interface with two sampling loops or enrichment columns. The comprehensive LC×LC system was introduced long ago,9 but practical applications have only started recently. Various experimental set-ups can be used for 2D LC×LC systems:
1. "Pseudo-multidimensional" LC×LC separations can be achieved on a single column by using sequential applications of different selective mobile phases.10
2. Two columns connected in series (or a single column with a mixed-stationary phase) and a single mobile phase can be used, for example, for SEC and normal phase (NP) or a non-aqueous RP separation of synthetic polymers; or for combined achiral and chiral separations.
3. A combination of two different columns using different separation modes in the first and in the second dimension, connected via a ten-port or a twelve-port switching valve (or several six-port switching valves) and one or more mobile phases (isocratic or gradient elution) can be used either in heart-cutting or in comprehensive LC×LC. Another recently reported variation, which combines this set-up and set-up 1, employs two columns with the same stationary phase (monolithic C18 columns), but different mobile phases in each dimension: tetrahydrofuran–water and methanol–water, respectively.11
4. A single column in the first dimension and two identical alternating columns in the second dimension used instead of the sampling loops in the comprehensive mode.
5. A single column in the first dimension may be connected to a single column in the second dimension via a switching valve where the sampling loops are substituted with two small trapping columns intended to retain (focus) sample contained in the effluent from the first column in a narrow zone and to reduce the volume of the fractions transferred to the second dimension. This is in principle a special case of three-dimensional (3D) LC×LC×LC set-up.
Development of 2D Separation Methods
The development and optimization of a fully automated comprehensive LC×LC separation is more difficult than that of a heart-cut technique because the separation of a fraction transferred into the second dimension should be accomplished within the time the next fraction from the first-dimension column effluent is collected in the sampling loop. This requirement sets limits to the volume of the sampling loops collecting the fractions from the first dimension, to the fraction transfer cycle frequency and to the resolution in the second dimension. Ideally, every peak from the first dimension should be transferred to the second dimension in at least three or four consecutive fractions. The maximum allowable pressure in both dimensions determines the time of a comprehensive LC×LC analysis.
The sequence of columns has a marked effect on the results of a 2D separation. For comprehensive LC×LC, the column with a higher peak capacity should be used in the first dimension system. A long narrow-bore column or a microcolumn and a low flow-rate of the mobile phase should be preferred in the first dimension, if allowed by the detection sensitivity. In the second dimension, a short and efficient column with an inner diameter equal to — or larger than — that used in the first dimension should be used at a high flow-rate for fast separation. Short columns packed with small particles (3 μm or less), non-porous, superficially porous or monolithic columns are especially suitable for this purpose.
The selection of the mobile phases in the first and in the second dimensions is very important, because the whole stationary/mobile phase system controls the retention and the separation selectivity. If possible, the mobile phase used in the first dimension should have low elution strength in the second dimension to focus the fraction transferred from the first dimension in a narrow zone on the top of the second-dimension column before the elution step. Of course, the mobile phases used in the two dimensions should be compatible.
Because of a higher peak capacity, gradient elution is generally preferred to isocratic LC for separations in the first dimension. Temperature programming offers another possibility for increasing peak capacity, especially in the separation of macromolecular or oligomer compounds.12 In the second dimension, the time necessary for the re-equilibration of the column before the transfer of the next fraction decreases the time available for the separation if repeated gradients are run with each transferred fraction. This problem can be avoided in some cases by using parallel gradients spanning over the whole gradient time both in the first and in the second dimension.13 If the second-dimension gradient run over the whole 2D separation time is shallow enough, the separation conditions are quasi-isocratic and do not cause problems in the evaluation of the 2D data.
2D Separations of Ordered Samples
Some "ordered" samples may contain structurally similar analytes, for example, homologues with different lengths of alkyls, polymers or oligomers with different numbers of repeat monomer units (either polar or non-polar ones),14,15 oils, fats or carotenoids with different numbers of carbon atoms and double bonds, etc. In many separation systems the repeat structural units show additive contributions to the retention (expressed in terms of the logarithms of the retention factors, k).16 In such a situation, the retention of compounds containing n repeat structural units is described by Equation 4:17
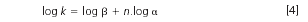
α = kj/ki is the relative retention, which is a measure of the separation selectivity for two compounds i and j differing by one repeat structural unit, Δn = 1, with retention factors ki and kj, respectively. β in Equation 4 is the contribution of the end-group(s) to the retention factor.
Equation 4 can be applied to describe the effect of the dual-type distribution of repeat structural units A and B in a "2D" sample on the retention selectivity in two LC systems, 1 and 2:
System 1:
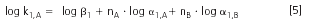
System 2:

Usually, the retention increases with increasing number of both types of structural units, A and B, but in some systems, a repeat structural unit may decrease the retention (α < 1); for example the retention of fatty acid derivatives in RP systems decreases with increasing number of double bonds.18 A 2D sample exhibits a disordered overlapping peak distribution in 1D separation systems because each of the two repeat units A and B contributes more or less to the retention, but shows a more or less ordered distribution of peaks in 2D separation systems.1
2D separation systems can provide detailed information on the bimodal distribution of repeat structural units in the samples of synthetic or natural polymers. For maximum gain in peak capacity, the LC×LC separation systems should provide good resolution for one type of distribution in the first dimension and for another type of distribution in the second dimension and in both dimensions suppress the differences in the retention because of the other type of distribution. In such 2D systems, the separation selectivities for various repeat structural groups are non correlated: α1,A 1, α2,A = 1 for A and α1,B = 1, α2,B 1 for B.18
In an appropriately designed non-correlated orthogonal 2D system, every fraction transferred from the first dimension contains only species with equal numbers of A units, nA, and second dimension yields the separation of species with different numbers nB.
It is often difficult to find fully orthogonal 2D LC systems because the separation systems that can be used in one or in both dimensions are more or less selective with respect to the distribution of the two structural elements A and B. To select suitable LC×LC systems with a high selectivity for one type of the repeat structural element in the first dimension and for the other structural element in the second dimension, the impact of the dual distribution on the retention in the two potentially useful separation systems should be taken into account.
The selectivity correlation for each structural element can be determined by comparing the retention of sample compounds with different numbers of the elements, A or B, in the first (1) and second (2) dimension separation systems by plotting log k1versus log k2. By rearranging Equation 5 and Equation 6 we obtain:
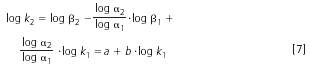
The coefficient b of Equation 7 provides a measure of the selectivity correlation for each repeat structural element in the two separation systems (b = 0 in non-correlated, i.e., orthogonal systems, b = 1 in identical or highly similar systems, i.e., low value of b indicates that the two systems are suitable for LC×LC separations of samples with bi-modal distribution).
Selecting Columns and Conditions
The crucial step in the development of a 2D LC×LC separation is the selection of the first- and second-dimension separation systems with non-correlated separation selectivities providing maximum gain in the peak capacity, which should be as close as possible to an orthogonal system with non-correlated selectivities (α in Equation 1 and Equation 2). Various combinations of RP and NP, ion-exchange (IEC), or size-exclusion (SEC) LC modes can suit specific separation problems, based on the differences in the molecular structure of separated compounds, such as size, polarity and shape, or the specific charge of ionic compounds.19, 20
Separations in RP and NP systems use the differences in the polarities and lipophilities of the analytes, but the size of the molecules and their acidity/basicity also contribute more or less to the retention in the RP and NP systems.
The polar separation selectivity is based on the differences in the selective forces controlling the retention, such as the dipole–dipole or proton-donor/acceptor interactions, which depend on the combination of the stationary and the mobile phases used. The retention in ion-exchange systems is generally controlled by electrostatic ion-exchange interactions, but can be more or less affected by non-ionic interactions with the stationary phase matrix. The mixed character of the real world interactions is the main reason why the separation selectivities in different systems can be more or less correlated, which should be taken into account when developing 2D LC×LC separations. The applications of RP×NP and RP×RP systems for 2D comprehensive separations were recently reviewed. 21
A combination of RP and NP systems can be used for 2D separations of samples with two or more types of structural elements differing in lipophilic and polar properties. In RPLC, a constant increase in retention often occurs with a regular increase in the number n of lipophilic repeat units such as methylene groups, n(CH2), whereas polar repeat units cause a regular increase in log k in NP systems. Successful selection 2D systems requires effective use of selective interactions between the analytes and the stationary and the mobile phases in different RP and NP separation systems, which can be evaluated on the basis of Equation 7 using the 2D retention data plots for suitable test samples differing in the number of various repeat units or other characteristic structural properties. Figure 2 shows examples of such correlation plots for natural antioxidants including phenolic acids (compounds 1–6, 9, 10, 12) and flavones (compounds 13–31).
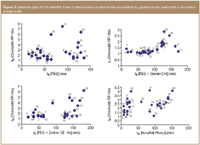
Figure 2
2D combinations of RPLC and ion-exchange (IEX) LC, with either salt concentration or pH gradients, are often used for separations of ionic compounds, acids or bases. SEC is often combined with NP or RP for 2D separations of synthetic polymers and biopolymers to provide separations based on the molar mass distribution (MMD) in one dimension and on the polarity (or lipophility) of the end groups in the other.
Compatibility of Phase Systems
Aqueous–organic mobile phases are the most common in RP chromatography, except for separations of strongly lipophilic compounds. On the other hand, NP chromatography employs polar adsorbents (or polar bonded stationary phases) with mixed purely organic mobile phases, as even traces of water may deactivate the adsorbent and destroy the separation.
In off-line 2D LC×LC, the fractions collected from the first dimension can be treated by evaporation, dilution or extraction before injection to the second-dimension column to suit the mobile phase. The compatibility of mobile phases in the first and in the second dimension is very important in designing a heart-cut and even more a comprehensive on-line set-up combining RP and NP separations. The transfer is usually feasible from an NP to a non-aqueous or high-organic RP system.
For example, carotenoids in orange essential oil can be separated by comprehensive LC×LC with a microbore silica gel column (Supelcosil LC-Si, Supelco, Bellefonte, Pennsylvania, USA) and a gradient of ethanol in hexane in the first, NP dimension and a monolithic C18 column (Chromolith Performance, Merck. Darmstadt, Germany) with a gradient of acetonitrile in a propanol–water mobile phase in the second, non-aqueous RP dimension.22 In another recent work, a micro-bore diol column (Beta-sil Diol, Thermo Electron, Cheshire, UK) with gradient of ethyl acetate in hexane in the first dimension was applied for the analysis of furocoumarins in lemon or orange oil extracts. Here, high proportion of hexane in the fractions transferred from the first (NP) dimension and gradients starting in pure water in the second (RP) dimension efficiently suppressed band broadening and distortion caused by mobile phase incompatibility in the two dimensions.23
On-line transfer from an RP to an NP system is more difficult, because water contained in the fractions transferred from the RP system may deactivate the polar stationary phase and destroy the separation in the second, normal-phase dimension. This problem can be avoided by using non-aqueous RP in the first dimension, if the sample compounds are lipophilic.
With polar sample compounds, hydrophilic interaction chromatography (HILIC) can be used in the second dimension, usually on stationary phases less polar than silica gel, for example aminopropyl silica, or specific bonded stationary phases, such as aspartamide, which allow the use of mobile phases containing up to 70% water.
Mobile phases used in RP×RP, RP×IEC, RP×SEC or NP×SEC 2D LC separation systems are usually fully miscible and have similar physicochemical properties. However, mobile phases with great differences in viscosities (such as acetonitrile–water and methanol–water) should be avoided in 2D systems, otherwise flow instabilities may occur because of a complex mixing of the two fluids in a pattern that resembles fingers (viscous fingering), resulting in considerable broadening or distortion of the peak shape of compounds separated in the second dimension.24
Another problem which may be encountered when designing a suitable RP×RP, RP×IEC, or RP×SEC 2D system is that a relatively high concentration of the organic solvent should be sometimes added to the first-dimension mobile phase for full recovery of the solutes, which however may have too high elution strength in the second dimension RP system and impairs the transfer of the solutes between the two dimensions. As a rule, the sample should be more strongly retained in the second than in the first dimension, to avoid excessive peak broadening and poor resolution in the second dimension. Last but not least, compatibility of the mobile phase with MS detection has to be observed if a 2D separation system is coupled with mass spectrometry.
SEC×NPLC and SEC×RPLC 2D LC×LC
Separations of macromolecular compounds: 2D separations of polymers often combine size-exclusion chromatography (SEC) for the separation according to the distribution of molar masses in one dimension and normal-phase, aqueous–organic or non-aqueous RP systems (depending on the polarity of the sample) in the other dimension under "near-critical" conditions, where compounds differing in the numbers of monomer units co-elute in a single peak.25 2D SEC×NPLC has been applied mostly to the separation and characterization of synthetic polymers, copolymers or polymer blends that are soluble in organic solvents, such as functional polystyrene (PS) and polymethyl methacrylate (PMMA) polymers, styrene-methacrylate (SMA) copolymers, styrene-butadiene star (PBS) polymers, butyl acrylate (BA) grafted onto polystyrene-butadiene (PSB), methyl methacrylate (MMA) grafted onto polybutadiene (PB) or onto ethane-propene-diene (EPD) rubber copolymers, epoxy resins, or styrene-methyl methacrylate (SSMA)-grafted natural rubber. In the NP dimension, usually a silica gel or cyanopropyl-modified silica column is used with tetrahydrofuran–cyclohexane, dichoromethane–heptane, dichloromethane–acetonitrile, dichloromethane–methanol, trichloromethane–cyclohexane, and so on, mixed mobile phases, either with isocratic or with gradient elution, whereas THF is most frequently used as the SEC mobile phase.
Conventional SEC columns are usually used in the first dimension as they are relatively long and the SEC analysis requires relatively long time, whereas an NP (or RP) column is used in the second dimension for separation of heart-cut fractions from the SEC column. Comprehensive LC×LC using short SEC columns (50 mm or less) for fast separations in the second dimension and a longer NP or RP column in the first dimension may be used to improve the separation of polymers according to the chemical composition (end group) distribution.25
Proteins, peptide fragments, or other biopolymers are often separated by SEC in the first dimension and RP chromatography in the second dimension. For example, peptides in tryptic digests of ovalbumin and serum albumin were separated by comprehensive LC×LC using SEC for the separation according to the molar mass distribution (MMD) in the first dimension and RP chromatography with two alternating non-porous, 1.8 μm C18 columns (Micra Scientific, Northbrook, Illinois, USA) in parallel set-up for fast second-dimension RP separation.26 Partial separation of humic substances using the heart-cut approach with a BioSep-S2000 SEC (Phenomenex, Tottance, California, USA) column in the first dimension and a Synergi polar RP column (Phenomenex) in the second dimension represents another 2D RPSEC application example.27
RP×IEX and SEC×IEX 2D LC×LC
Separations of low- and high-molecular compounds: The retention in IEC is controlled principally by the solute charge, so that the IEX separation mechanism differs from the SEC and RPLC. However, it was shown recently that neither RP×IEX nor SEC×IEX 2D systems are fully orthogonal, presumably because of hydrophobic interactions with the non-polar moieties in the ion exchange stationary phases.28 Combinations of an IEX column in the first dimension and of a size-exclusion column in the second dimensions were used for 2D separations of peptides and proteins.29 However, the SEC mode generally has limited peak capacity and, furthermore, the diol columns often used for SEC in aquoeus mobile phases may retain some peptides and reduce their recovery, unless mobile phases with high enough concentrations of acetonitrile are used.
Combinations of ion-exchange (solvent or pH gradient) separations in the first dimension and RP separation in the second dimensions are useful for 2D LC×LC of weakly acidic or basic compounds. A fine example is the comprehensive LC×LC analysis of low-molecular mono- and dicarboxylic acids in atmospheric aerosols using a microbore strong cation exchange column (SCX) (Hamilton, Reno, Nevada, USA) in the ion-exclusion mode in the first dimension and a short monolithic or packed (2.5 μm particles) C18 column (Waters, Milford, Massachussets, USA) in the second dimension.30
For RP×IEX 2D separations of peptides and proteins or biological amines, 2D systems employing a microbore anion-exchange (SAX) or a cation-exchange (SCX) column with a salt concentration gradient (step or continuous) in the first dimension and a RP column31 in the second dimension are often used. RP×SCX 2D systems are most easy to use with increasing ionic strength gradients to elute peptides from the first dimension SCX column and transfer it onto the second RP dimension. Fast comprehensive LC×LC separation of bovine serum albumin tryptic digests with the total peak capacity of 1350 in 20 min was achieved recently by using a 20 s high-temperature binary gradient at 100 °C on a short stable-bond C18 microcolumn in the second dimension after first-dimension separation on an SCX microcolumn (Agilent Technologies, Palo, Alto, California, USA).32
Some hydrophobic peptides may be incompletely recovered or elute with poor peak shape from the first-dimension column as a result of non-specific interactions with the matrix of ion exchangers. The elution can be enhanced by adding organic solvents to the mobile phase, but the aqueous–organic solvent decreases the retention of peptides on the 2D RP column, which may affect the on-line fraction transfer to the second dimension.
Proteins can be separated according to their isoelectric points on an ion-exchange column using pH gradients in the first dimension and according to their hydrophobicity on an RP column in the second dimension. This 2D technique may offer a fast alternative to 2D capillary electrophoresis in proteomic research, because it is fast and can be easily automatized,33 even though the design of truly linear pH gradient requires some efforts.34 Recently, orthogonal 2D separations on a strong cation-exchange column in the first dimension and RP column on a microfluidic HPLC chip in combination with MS were applied to characterize the proteomes.35
NP×RP 2D LC×LC Separations of Ordered Samples
Surfactants and triacylglycerols: 2D separations of alcohol ethoxylate surfactants were achieved using gradient NPLC for the separation according to the distribution of the oxyethylene (EO) units in the first dimension and isocratic RPLC with 95% organic mobile phase for the separation according to the distribution of alkyl chain lengths in the second dimension.36 To optimize the separation of synthetic EO-PO block co-oligomer surfactants with dual distribution of oxyethylene (OE) and oxypropylene (OP) monomer units, selectivity correlations in various RP and normal-phase systems were evaluated.
Whereas C18 columns in the first dimension provide satisfactory separation according to the PO distribution and show low selectivity for the separation according to the number of EO units, normal-phase systems show lower separation selectivity for the distribution of PO units than of EO units.37 Aminopropyl silica columns provide better separation according to the EO distribution than bare silica gel or other polar-bonded phase columns.
The combination of a C18 microcolumn (Agilent Technologies) with a shallow gradient of acetonitrile in water in the first dimension and an aminopropyl microcolumn (Separon SG-Amin, Tessek, Prague, Czech Republic) with a ternary ethanol– dichloromethane–water mobile phase for NP HILIC separation in the second dimension provides a 2D system with a high degree of orthogonality, allowing on-line comprehensive LC separation of EO-PO co-oligomers according to the PO units distribution in the first dimension and separation according to the EO units distribution in the second dimension.
Figure 3 shows a contour plot of a comprehensive RPLC–NPLC separation of EO-PO co-oligomers with 1–2 EO units and polyethylene glycol (PEG) and polypropylene glycol (PPG) homopolymers. No mobile phase incompatibility problems were observed with 10 μl fractions transferred from the first to the second dimension.38 The HILIC mode with a bare silica gel column in the second dimension combined with the RP mode in the first dimension was reported to provide improved orthogonality for the separation of peptides in comparison to the RP×SCX 2D LC×LC separations.28

Figure 3
Natural fat and oil samples containing saturated and unsaturated mono-, di- and tri-acylglycerols (TAGs) of fatty acids are typical multidimensional samples with different numbers of carbon atoms, nC, and double bonds, nDB. Different positions of the double bonds and occurrence of cis- : trans- : sn1 : sn2 : sn3 isomers adds additional sample dimensionalities.39 A strong inverse correlation between the selectivities for the distribution of double bonds and acyl lengths in RP systems was observed: a structural increment of one double bond decreases the retention of acylglycerols approximately in the same way as the structural decrement of two methylene groups in a saturated compound; hence co-elution of TAGs with the same "equivalent carbon numbers", ECN = nC – 2nDB, is observed.
In spite of this selectivity correlation, "pseudo-2D" LC×LC two-step ternary RP gradients with aqueous–organic mobile phase in the first step and a non-aqueous gradient in the second step may separate some TAGS with the same ECNs but different numbers of double bonds on efficient C18 columns with non-endcapped residual silanol groups,40 because the degree of the double-bond and the acyl length selectivity depends on the stationary phase properties.41
In 2D LC×LC using the non-aqueous (NA) RPLC combined with argentation (silver-ion) NP chromatography the number of resolved TAG species largely improves. The separation in silver-ion (argentation) normal-phase (AgNP) mode is based on the differences in π–π electron interactions with the silver ions, resulting in the separation of species differing in the number, position and geometrical configuration of double bonds. AgNP columns can be prepared by flushing silica gel columns with aqueous solution of silver nitrate, but best results are achieved using commercial "lipids" columns. Organic mobile phases with low concentrations of polar solvents (e.g., hexane–acetonitrile) are usually employed in the isocratic mode.
Off-line coupling of a C18 column (Ultra C18, Restek, Sanderton, UK) with non-aqueous mobile phase (acetone–acetonitrile) in the first dimension and an AgNP column (Chromospher 5 Lipids Superchrom, Milan, Italy) with a hexane–acetonitrile mobile phase in the second dimension considerably increases the number of triacylglycerols identified of vegetable oils.42 Comprehensive LC×LC 2D separations are also possible with the silver-ion chromatography in the first dimension under gradient conditions and fast non-aqueous RP on a monolithic column with isopropanol–acetonitrile mobile phases in the second dimension.43,44
Separations of Non-ordered Samples
2D separations using alkyl silica gel in one dimension and stationary phases with specific ligands in the second dimension may show low selectivity correlation and a high degree of orthogonality for separations of some complex samples containing compounds differing in size, polarity and acidity. Chromatographic methods that can be used for the characterization of the retention and selectivity of columns for RP chromatography were reviewed recently.45 In an extensive study, five different selectivity parameters characterizing hydrophobicity, steric resistance to penetration, hydrogen bond acidity and basicity and cation-exchange capacity were determined for more than 350 commercial HPLC columns on the basis of the retention data for 67 test compounds with large differences in selectivities.46 The hydrophobic subtraction model based on the comparison of these parameters provides a measure of column similarity or equivalency, which can be used as a useful clue for the selection of suitable combinations of various silica-based RP columns, temperature and solvent gradients for RP×RP 2D LC×LC separations of drugs and degradation products.47
RP×RP 2D systems were used in practice for the analysis of complex food, natural extracts, pharmaceutical and environmental samples. For example, barbiturates and other drugs were separated using hybrid silica–organic support X-Terra MS C18 column (Waters) in the first dimension and two SB-Phenyl columns (Agilent Technologies) in parallel alternating set-up in the second dimension.48 Active medicinal constituents from natural plant extracts were separated using heart-cutting technique on a semi-preparative bonded nitrile column (Phenomenex) in the first dimension and a C18 column (Phenomenex) in the second dimension, both with an acetonitrile–water mobile phase.49
A 2D system using specific interactions on an affinity chromatography (AC) column with human serum albumin bonded on silica gel in the first dimension and a monolithic C18 column (Chromolith, Merck, Darmstadt, Germany) in the second dimension was applied for comprehensive LC×LC analysis of natural extracts containing traditional Chinese medicines.50
The individual amino acids contained in various peptides show different contributions to the retention and selectivity of separation on various RP columns.51 Hence, peptides in tryptic digests could be separated using either two different mobile phases and a single RP capillary column, or a single mobile phase, but two capillary columns packed with different stationary phases.52 RP×RP 2D chromatography coupled with MS was suggested for separation of hydrophobic C-peptides lacking positively charged amino acids, which precludes using cation-exchange chromatography (CAC) for the separation in one dimension. A Jupiter C5 (Phenomenex) and gradient elution with mobile phases containing 0.1% trifluoroacetic acid (TFA) were used in the first dimension, whereas a Polaris C18 column (Varian, Lake Forest, California, USA) preflushed with a mobile phase with low concentration of organic solvent and an independent gradient elution without TFA in the second dimension. In this set-up, peptides transferred from the first dimension were pre-concentrated on the second-dimension column and introduction of MS-unfriendly TFA into the mass spectrometer was avoided.53
Various stationary phases, including phenyl,54 or PEG ligands bonded on silica,55 or zirconium dioxide support with deposited a carbon layer56 provide significantly different separation selectivity for phenolic and flavone natural antioxidants compared with octadecylsilica (ODS)-bonded columns and can be used for 2D RPXRP LC of this important class of compounds. Figure 2 illustrates the orthogonality of PEG/C18–silica (Supelco) and phenyl/C18-silica systems (Waters), (non-correlated plots of retention factors) for phenolic compounds. Serially coupled PEG and C18 columns show significant improvement in resolution of phenolic antioxidants in comparison to a single PEG column in the first dimension.55
A PEG-silica (Supelco) or a phenyl-silica column (Waters), or serially coupled PEG (Supelco) and C18 packed columns (Merck) in the first dimension and a monolithic C18-silica column (Merck) in the second dimension can be employed for comprehensive LC×LC separations of phenolic antioxidants in beverages and plant extracts. Figure 4(a), (b) show a contour graph and a 3D chromatogram representation of comprehensive 2D separation of natural antioxidants with a bonded phenyl column columns (Waters) in the first dimension and a monolithic C18 column columns (Merck) in the second dimension, using two short X-terra trapping columns (Waters) in a ten-port switching valve interface for fraction transfer modulation and parallel acetonitrile gradients in ammonium acetate buffer (pH = 3) in the two dimensions.13
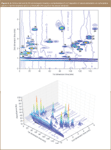
Figure 4
The selectivity of a zirconia–carbon column (Supelco) for natural antioxidants differs very significantly from the selectivities of bonded C18, PEG or phenyl columns and can be used for orthogonal comprehensive separations of phenolic compounds. The zirconia-based columns are stable at high temperatures; high-temperature operation improves the speed of analysis in the second dimension. Furthermore, the mobile phases used with silica-based columns generally have lower elution strength with zirconia columns, so that the transferred fractions from the first dimension are adsorbed in a narrow zone of a zirconia–carbon column. Hence, a C18 microcolumn (Supelco) in the first dimension and two alternating zirconia–carbon columns (Supelco) in the second dimension could be used for comprehensive LC×LC separations of phenolic antioxidants in beer and wine;50 unfortunately flavones are retained too strongly on the zirconia column even in acidified organic solvents and cannot be separated under these conditions. Fast comprehensive RP×RP 2D separation of 140 indole-acetic acid derivatives and other compounds in maize extracts was accomplished in approximately 30 min using a pentafluorophenylpropyl (PFPP) column (Supelco) with a gradient of acetonitrile in sodium phosphate–sodium perchlorate buffer in the first dimension and a short carbon-coated zirconia column (Supelco) with a high temperature (110 °C) fast (21 s) gradients of acetonitrile in water with 0.02 M perchloric acid in the second dimension.57
Acknowledgements
This work was partly supported by the by the Ministry of Education of Czech Republic, project No. MSM0021627502and by the Grant Agency of Czech Republic, project No. 203/07/0641.
Pavel Jandera PhD, DSc is professor in Analytical Chemistry and team leader of the group of analytical separations at University of Pardubice, Czech Republic. He is the member of the editorial boards of Journal of Chromatography A and Analytical Letters. He is specialist in development and optimization of programmed separation techniques.
References
1. J.C. Giddings, J. Chromatogr. A, 703, 3–15 (1995).
2. J.C. Giddings, Anal. Chem., 39, 1027–1028 (1967).
3. M. Martin, D.H. Herman and G. Guiochon, Anal. Chem., 58, 2200–2207 (1986).
4. J.M. Davis, Anal. Chem., 63, 2141–2152 (1991).
5. J.M. Davis, Anal. Chem., 65, 2014–2023 (1993).
6. U.D. Neue et al., Adv. Chromatogr., 41, 93–136 (2001).
7. L.R. Snyder, J.W. Dolan and J.R. Gant, J. Chromatogr., 165, 31–58 (1979).
8. P. Jandera and J. Churáãek, J. Chromatogr., 91, 207–221 (1974).
9. F. Erni, R.W. Frei, J. Chromatogr., 149, 561–569 (1978).
10. E.L. Little, M.S. Jeansonne and J.P. Foley, Anal. Chem., 63. 33–44 (1991).
11. T. Ikegami et al., J. Chromatogr. A, 1106, 112–117 (2006).
12. K. Im et al., Anal. Chem., 79, 1067–1072 (2007).
13. F. Cacciola et al., J. Chromatogr. A, 1149, 73–87 (2007).
14 P. Jandera, M. Holãapek and L. Kolá½ová, Int. J. Polym. Anal. Charact., 6, 261–294 (2001).
15. T. Chang, Adv. Polym. Sci., 163, 1–60 (2003).
16. A.J.P. Martin, Biochem. Soc. Symp., 3, 4 (1949).
17. P. Jandera, J. Chromatogr., 314, 13–36 (1984).
18. P. Jandera et al., J. Chromatogr. A, 1087, 112–123 (2005).
19. R.A. Shellie and P.R. Haddad, Anal. Bioanal. Chem., 38, 405–415 (2006).
20. S.P.Dixon, I.D. Pitfield and D. Perrett, Biomed. Chromatogr., 20, 508–529 (2006).
21. P. Jandera, J. Sep. Sci., 29, 1763–1783 (2006).
22. P. Dugo et al., Anal. Chem., 78, 7743–7750 (2006).
23. I. Francois, A. de Villiers and P. Sandra, J. Sep. Sci., 29, 492–498 (2006).
24. K.J. Mayfield, J. Chromatogr. A, 1080, 124–131 (2005).
25. A. Van der Horst, and P.J. Schoenmakers, J. Chromatogr. A, 1000, 693–709 (2003).
26. G.J. Opiteck, J.W. Jorgenson and R.J. Anderegg, Anal. Chem., 69, 2283–2291 (1997).
27. I.J. Whelan et al., Ind&Eng Chem Research, 44, 3229–3237 (2005).
28. M. Gilar et al., Anal. Chem., 77, 6426–6434 (2005).
29. M.M. Bushey and J.W. Jorgenson, Anal. Chem. 62, 161–167, (1990).
30. J. Pol et al., J. Chromatogr. A, 1130, 64–71 (2006).
31. L.A. Holland and J.W. Jorgenson, J. Microcolumn Sep., 12, 371–377 (2000).
32. D.R. Stoll, P.W. Carr, J. Am. Chem. Soc.,127, 5034 –5035 (2005).
33. M. Pepaj et al., J. Sep. Sci., 29, 519–529 (2006).
34. T. Anderson et al., J. Chromatogr. A, 1024, 217–226 (2004).
35. M. Vollmer et al., J. Sep. Sci., 29, 499–509 (2006).
36. R.E. Murphy, M.R. Schure and J.P. Foley, Anal. Chem., 70, 4353–4360 (1998).
37. P. Jandera et al., Chromatographia, 60, S27–S35 (2004).
38. P. Jandera et al., J. Chromatogr. A, 1119, 3–10 (2006).
39. M. Holãapek et al., J. Chromatogr. A, 858, 13–31 (1999).
40. M. Holãapek et al., J. Chromatogr. A, 1010, 195–215 (2003).
41. P. Jandera, M. Halama and K. Novotná, J. Chromatogr. A, 1030, 33–41 (2004).
42. P. Dugo et al., J. Chromatogr. A, 1041,135–142 (2004).
43. P. Dugo et al., Anal. Chem., 76, 2525–2530 (2004).
44. L. Mondello et al., J. Chromatogr. A, 1086, 91–98 (2005).
45. P. Jandera and K. Novotná, Anal. Letters, 39, 2095–2152 (2006).
46. L.R. Snyder, J.W. Dolan and P.W. Carr, J. Chromatogr. A, 1060, 77–116 (2004).
47. J. Pellett et al., J. Chromatogr. A, 1101, 122–135 (2006).
48. C.J. Venkatramani and A. Patel, J. Sep. Sci., 29, 510–518 (2006).
49. V. Wong and A. Shalliker, J. Chromatogr. A, 1036, 15–24 (2004).
50. L. Hu et al., J. Sep. Sci., 29, 881–888 (2006).
51. T. Baczek, J. Sep. Sci., 29, 547–554 (2006).
52. J.P.C. Vissers et al., J. Microcolumn Sep., 11, 277–286 (1999).
53. E. Rogatsky et al., J. Sep. Sci., 29, 529–537 (2006).
54. T.J. Whelan et al., J. Chromatogr. A, 1097, 148–156 (2005).
55. F. Cacciola et al., J. Sep. Sci., 29, 2500–2513 (2006).
56. F. Cacciola, P. Jandera and L. Mondello, J. Sep. Sci., 30, 462–474 (2007).
57. D.R. Stoll, J.D. Cohen and P.W. Carr, J. Chromatogr. A, 1122, 123–137 (2006).

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.



















